
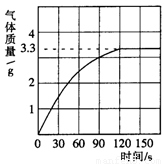
��3�֣�ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10����������뵽12.5gˮ���У�����CO2������������ͼ��ʾ��
��������ʾ��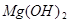 +2HCl=
+2HCl=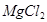 +H2O��
+H2O��
��1����ͼ�п��Կ�����12.5gˮ�������ᷴӦ�����ɵĶ�����̼����� g��
��2��ˮ����̼��Ƶ����������Ƕ���?
��3������ˮ���г�̼��ƺ�������þ�⣬�������������ʣ��ܽ�12.5 gˮ����������Ҫ��������Ϊ10��������������� (���������һλС��)��
��1��3.3g����2��60%����3��117.7g
��������
�����������1����ͼ�п��Կ�����12.5gˮ�������ᷴӦ�����ɵĶ�����̼�����3.3g��
��2����12.5gˮ����̼��Ƶ�����Ϊx
CaCO3+ 2HCl==CaCl2+H2O+CO2��
100 44
x 3.3g
100��44=x��3.3g
x =7.5g
ˮ����̼��Ƶ���������Ϊ��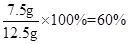 ��
��
��3��12.5 gˮ���к���̼���7.5g������������þ12.5-7.5=5g��
�跴Ӧ̼��Ƶ�ϡ���������Ϊm����Ӧ������þ��ϡ���������Ϊn��
CaCO3+ 2HCl==CaCl2+H2O+CO2��
100 73
7.5g m��10%
100��73=7.5g��m��10%
m=54.8g
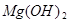 +2HCl=
+2HCl=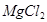 +H2O
+H2O
58 73
5g n��10%
58��73=5g��n��10%
n=62.9g
���ܽ�12.5 gˮ����������Ҫ��������Ϊ10���������������54.8g+62.9g=117.7g��
��12.5gˮ�������ᷴӦ�����ɵĶ�����̼�����3.3g��ˮ����̼��Ƶ�����������60%���ܽ�12.5 gˮ����������Ҫ��������Ϊ10���������������117.7g��
���㣺���ݻ�ѧ����ʽ���㣻��ѧ��Ӧ�е�������ϵͼ�������ij�ɷֵ�����������
���������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢�𡣻������ij�ɷֵ���������= ��
��


 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ��
��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ��
ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ⶨʱ�䣨���ӣ� | 0 | 1 | 2 | 3 |
| pH | 4.73 | 4.62 | 4.56 | 4.55 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ��
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com