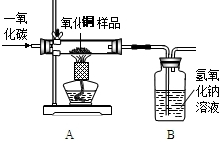
��ͼΪʵ�����г����������Ʊ������������ʵ��IJ���������
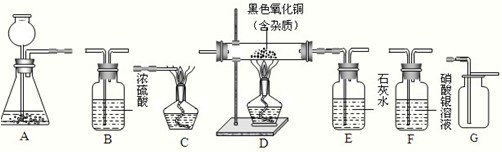
��ش��������⣺
��1����ʵ�����ô���ʯ��ϡ���ᷴӦ��ȡ���ռ�һƿ����Ķ�����̼��
����ѡ����������˳��Ϊ
A��F��B��G
A��F��B��G
����д���������ĸ����
�����ɶ�����̼�Ļ�ѧ����ʽΪ��
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��
��2������һ������壬����Ҫ�ɷ�ΪCO����������ˮ�����������ܺ�������HCl���壮ij��ѧ�������ͬѧ���øû���������ʵ�飺
�ټ���HCl�����Ƿ���ڣ�����ȥHCl���壻
���ø��﴿����CO���廹ԭ����������ͭ���������е����ʲ������仯����
��ʵ��CO���廹ԭ����ͭ���ɵ���������������ʣ�
������ͼ��ʾ��������װһ��ʵ��װ�ã��������ʵ������
��ʵ�����ʱ����Ҫ��������������������⼸���������������˳��Ӧ�ǣ���д����װ�ã���
F��B��D��E��C
F��B��D��E��C
��
���ж�HCl�����Ƿ���ڵĸ�����
F���Ƿ��а�ɫ��������
F���Ƿ��а�ɫ��������
��
������Ӧ������ȫ��������E����������a g��������D�е�������������
0.36a
0.36a
g��
����ԭ��������л��ж�����̼����Ӧ������ȫ����ͨ������E������������������Ʒ������ͭ�Ĵ��ȣ���������
ƫ��
ƫ��
���ƫС����ƫ������Ӱ�족֮һ����

 Cu+CO2������ش�������⣺
Cu+CO2������ش�������⣺
 Cu+CO2
Cu+CO2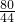 =
=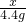
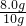 ��100%=80%
��100%=80%




 Cu+CO2������ش�������⣺
Cu+CO2������ش�������⣺