
���Ϲ���63���������2011����Ϊ���ʻ�ѧ�꣬�������ǡ���ѧ���������������δ��������ش����л�ѧ������������ص����⡣
��1����������һ�ֿ��ʳƷ���������ͼ��������Ϣ���ش��������⣺
| ��������Ӫ���ɷ� | |
| Ӫ���� | ÿ�ݺ��� |
| ������ | 29.6g |
| ��֬ | 23.5g |
| ���� | 104.7g |
| �� | 814mg |
| �� | 130mg |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���ѧ�������ߣ��������ǵ�����ϢϢ��أ���Ӣټ������ʯ�Ң�һ����̼������آ������а���ȷ�Ĵ�������ڶ�Ӧ�Ŀո��ڡ�
��1���������� �� ��2����������� ��
��3���������������������� �� ��4�������ڻ�ѧ����ʹ�õ��� ��
��5�����������ȼ�ϵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̼Ԫ��������������ʵĻ���Ԫ�ء�
��1�����к�̼Ԫ�ص������У������л������ ������ĸ��ţ���
A��̼���� B���Ҵ���C2H5OH �� C��������̼
��2���� 440���ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��3����̼���ࡱ����֪����Ĺ�����ϣ���ͼ��ʾ��100cm3�ġ�̼���ࡱ���ȡ��ڹ�β�Ͳ��ϣ���ϸ�IJ���һ�㶼û��ѹ�䣩������̼Ԫ����ɣ����ж�ṹ�����Ժá�����ʯ���к�ǿ����������������ˮ�����������ʯ�ͼ������Կɻָ�ԭ״�����й���̼�����˵����ȷ���� ������ĸ��ţ���
A������������ B�����ظ�ʹ�� C���ɴ�������ʯ��й©
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ش����������е��й����⣺
(1)��������ά����ë��ά�ͺϳ���ά����������� ʵ�顣
(2)��������Ҫ���е�Ӫ������ (����ࡱ�����ʡ�)���ܲ���ά����C��ʳ���� ��
(3)��ĥ�õĶ�������ɴ�����н����ͽ����룬�൱��ʵ������е� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�������ϰ������Ρ����㡢���⡢ʳ�Ρ����ܲ��ȣ����к��е�Ӫ�������� ������д���֣�����������ġ�Ӫ���ء��в������л�������� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����벻����ѧ����ѧ������ϢϢ��أ�
��1��������ʳ���潡��������ʳ���У����������ʵ����� ��������ĸ��ţ���
��2��Ϊ��ȫ��Ľ������ҹ��ƹ�ʹ��ǿ�������ͣ�����ġ�����ָ������ ��������ʡ�����Ԫ�ء����ӡ���������ȱ���������� ����
��3�������к����������������к��м����ֶ������к������ʣ�����Ŷ������͡�һ����̼��������̼�ȣ�������Ѫ�쵰�������ǿ���ж��������� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����벻����ѧ����ѧ������ϢϢ��أ�
��1��������Դ�ˮ�����߲�����ȡ�� ��������������Ҫ����С�������������³´�л��Ԥ��������ά�����彡�������ã�
| ʳƷ | ����֭ | ƻ��֭ | ����֭ | ����֭ | ţ�� | ������ |
| pH | 2.4 | 3.1 | 4.2 | 4.4 | 6.5 | 7.8 |
| Ӫ���ɷֱ� | ||
| Ӫ���ɷ� | ÿƬ���� | ÿ100g���� |
| þ | 6.7mg | 1.12g |
| Ҷ�� | 255 | 42.5mg |
 2Na2CO3+�� ����
2Na2CO3+�� �����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͥ��������һ����ѧС���磬������ư�衱�͡��������ν��ײ衱�а��������ѧ֪ʶ��
��1�����г�����Ʒ��ʹ�õ���Ҫ���ϣ����ڽ������ϵ�����������д��ĸ��ţ���ͬ���������л��ϳɲ��ϵ����� ����
��2��������Ȼ������Ҫ�ɷ��Ǽ��飬����ȼ�յĻ�ѧ����ʽΪ�� ����
��3��ʳ��������ʳ����ҪΪ���岹���Ӫ�������� ����
��4��ϴ�Ӽ�����ϴ�;��ϵ����ۣ�������Ϊϴ�Ӽ������� �����ܣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ�����������ߣ���ͨ��һ�����Σ�ijͬѧ����һ�������˸������ᣮ
��1������ǰ�������˼�������������ӺͿ�Ȫˮ�����и���ά���ص����� ����
��2�����ι����У������ù��Ŀ�Ȫˮƿ�ŵ��������� ������ɻ��ա����ɻ��ա�����ʶ���������ڣ�
��3��;��СϪ����ȡ������Ϫˮ�������м������ˮ�����۲쵽�� ������˵����ϪˮΪӲˮ��
��4���������������ܿ�����������ȫ��־���� ��������ĸ����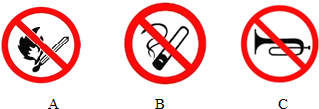
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com