
 ��ѧ�����ǵ�����ϢϢ��أ�������Ϊ����������˷������ʻ���������Ϊ�������ɳ�����չ�����˾��ף�
��ѧ�����ǵ�����ϢϢ��أ�������Ϊ����������˷������ʻ���������Ϊ�������ɳ�����չ�����˾��ף�| ��֤���������� | ���ܿ��������� | ���� |
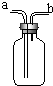
���� ��1����֤��������̼һ�����ó���ʯ��ˮ���������ʯ��ˮ���˵���ж�����̼���ڣ�����Ϊ�̼�Ӧ�ÿ��dzɱ�����ȫ�����õȷ��棻
��2���ٸ����������ܶȱȿ������ܶȴ���������ܣ�
�ڸ���������������ˮ��������ѹ��ˮ�ų�ȥ���������ܣ�
�۸���������������ˮ�����ͨ��ˮ�л�������ݷ�����
��� �⣺��1����С��������Ƕ�����̼��������̼�ļ���һ�����ó���ʯ��ˮ���������ʯ��ˮ����˵���Ƕ�����̼���ڣ� �ʴ�Ϊ��
| ��֤���������� | ���ܿ��������� | ���� |
| ��ע������ȡ��װ������������������ע�����ʯ��ˮ�� | ����ʯ��ˮ����� | �ð�װ�е������Ƕ�����̼ |
���� ���⿼���˶�����̼�ļ���װ�õ�ʹ�ã����������ó���ʯ��ˮ����������̼����ǵ��ص���������������ռ�����ȡ����������ܶȺ��ܽ��ԣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
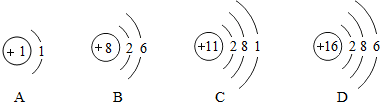
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��һ���л��� | B�� | ��̼���⡢��Ԫ����� | ||
| C�� | ̼���⡢��Ԫ�ص���������1��2��1 | D�� | ��Է�������Ϊ30 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ƿ | ��ƿ+ϡ���� | ��ƿ+��Ӧ����Һ | |
| �������ˣ� | 35.5 | 55.5 | 80.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
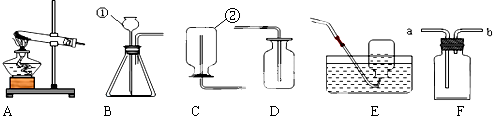
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Ŀ��� | |
| B�� | �����ߡ��ɴ��ӵ�����ת�������� | |
| C�� | ����ָ����ȷ���������� | |
| D�� | ���Ϳɽ������ϴ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����þ MgO2 | B�� | ������ AL2O3 | C�� | ̼���� NaCO3 | D�� | �������� SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�̪����ʳ��ˮ��ϡ���� | B�� | ��ˮ������ʯ�Һ�ʯ��ʯ | ||
| C�� | ��ȼ�ŵ�ľ����������Ͷ�����̼ | D�� | ���Ȼ�����Һ����ϡ�����ϡ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com