
���� ��1����Һϡ���������ʵ��������ֲ��䣻
��2���������ĵ���������ʵ������Ͷ�Ӧ�Ļ�ѧ����ʽ�����������Ƶ���������������������������
��3��������Ԫ���غ㣬�������������ƻ���̼�����е�������ת��Ϊ�������е��ƣ����������Ƿ�����Լ����ʵij̶���Σ����ĵ�������������䣮
��� �⣺
��1��������ˮ������Ϊx
98%��20g=10%����20g+x��
x=176g
��2��10%������49g�������ʵ�����Ϊ10%��49g=4.9g
���������Ƶ�����Ϊy
2NaOH+H2SO4=Na2SO4+2H2O
80 98
y 4.9g
$\frac{80}{98}$=$\frac{y}{4.9g}$
y=4g
��ˮ������NaOH����������Ϊ$\frac{4g}{80g}$��100%=5%
��3��������Ԫ���غ㣬�������������ƻ���̼�����е�������ת��Ϊ�������е��ƣ����������Ƿ�����Լ����ʵij̶���Σ����ĵ�������������䣮����ȡ80g����ˮ���ձ��з��ü���������е���������ȫ��ת��Ϊ̼���ƣ����ʱӦ��μ���10%������49�˲���ʹ̼����ǡ����ȫ��������ƣ�
�𣺣�1����20g����Ũ����ϡ��Ϊ��������Ϊ10%�����ᣬ��Ҫˮ�������� 176g��
��2����ˮ������NaOH������������5%��
��3��ȡ80g����ˮ���ձ��з��ü���������е���������ȫ��ת��Ϊ̼���ƣ����ʱӦ��μ���10%������49�˲���ʹ̼����ǡ����ȫ���������
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
 ʵ������һƿʧȥ��ǩ�Ĺ���������Һ��Ϊ�ⶨ������������������ȤС��ͬѧȡl00g����Һ��1g�������̻�ϣ�ʹ���ַ�Ӧ����������������ͷ�Ӧʱ���ϵ��ͼ��
ʵ������һƿʧȥ��ǩ�Ĺ���������Һ��Ϊ�ⶨ������������������ȤС��ͬѧȡl00g����Һ��1g�������̻�ϣ�ʹ���ַ�Ӧ����������������ͷ�Ӧʱ���ϵ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
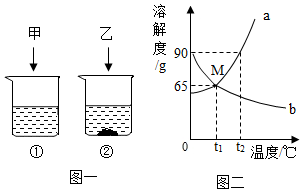 t2��ʱ�����ס��Ҹ�80g�ֱ����ʢ��100gˮ�������ձ��У�����ܽ�ָ���t2�棬������ͼһ�����ҵ��ܽ��������ͼ��������ͼʾ�ش��������⣺
t2��ʱ�����ס��Ҹ�80g�ֱ����ʢ��100gˮ�������ձ��У�����ܽ�ָ���t2�棬������ͼһ�����ҵ��ܽ��������ͼ��������ͼʾ�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | Ŀ�� | |
| A | �����˺�ij�����Һ�����������м������� | ��ȥ�����е����������� |
| B | ȡһС��ƹ������Ƭ����ֽ��Ƭ���ֱ�������ǯ��ס�����ھƾ��ƻ����ϼ��� | ֤��ȼ�յ�����֮һ��Ҫ������ |
| C | �����ȵ�ľ̿Ѹ������ʢ�������ļ���ƿ�ײ� | �۲�ľ̿��������ȼ�յ����� |
| D | ��ʢ��ˮ��С�ձ��м�������Ʒ�� | ֤��Ʒ���ھ��õ�ˮ�л���ɢ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
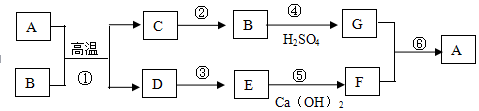
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ij��ˮ��pH��7�������ˮһ�������� | |
| B�� | ���˿��Գ�ȥˮ�в��������ʣ���˹��˺��ˮһ������ˮ | |
| C�� | һ����̼�Ͷ�����̼�����Ԫ����ͬ���������ǵĻ�ѧ������ͬ | |
| D�� | ��ȼ��ȼ��ʱ�¶���Ҫ�ﵽ�Ż�㣬�����¶ȴﵽ�Ż��ʱ����ȼ���һ����ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��һ������ϡ�����в��ϼ�����������Һ | |
| B�� |  ��һ���������������ͭ�����Һ�в��ϼ��Ȼ�����Һ | |
| C�� | 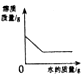 ��һ�������������Һ�в��ϼ�ˮ | |
| D�� | 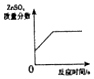 ��һ������ϡ�����в��ϼ�п�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ϴ����-�������黯���� | |
| B�� | �����������������-����������ˮ��Ӧ | |
| C�� | ϡ��������������-ϡ�����廯ѧ�����ȶ� | |
| D�� | ������̼���-������̼�ܶȱȿ���С |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com