
����Ŀ��ѧϰС��ƻ��ⶨ��������̼��Ƶĺ�����ȡ20g���������Թ��м��ȣ����ȹ��̲��ʣ�������������±���ʾ(���ʲ��μӷ�Ӧ��������Ԫ��)����ش���������:
ʱ��/s | 0 | 10 | 20 | 30 | 40 |
ʣ���������/g | 20 | 18 | 15.6 | 15.6 | m |
��1��m��ֵΪ_______g��
��2����������̼��Ƶ����������Ƕ���? (д���������)______
��3��������ʱ��Ϊ10sʱ��ʣ������и�Ԫ�ص�������______��
���𰸡�15.6 50% 4g
��������
̼����ڸ��������·ֽ����������ƺͶ�����̼����Ӧǰ��������Ϊ��Ӧ���ɶ�����̼�����������ݶ�����̼�������Լ���̼�����������һ�����Լ��㼦������̼��Ƶ����������ͷ�Ӧ��ʣ������и�Ԫ�ص�������
��1�����ݱ����е����ݣ����ȵ�20s֮��ʣ�������������ټ�С��m��ֵΪ15.6g��
��2��̼�����ȫ�ֽ�����������̼������Ϊ��20g-15.6g=4.4g��
��μӷ�Ӧ��̼��Ƶ�����Ϊx
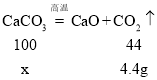
![]()
x=10g
��������̼��Ƶ�����������![]()
��3����ѧ��Ӧǰ��Ԫ���������䣬������ʱ��Ϊ10sʱ��ʣ������и�Ԫ�ص���������ԭ10g̼����и�Ԫ�ص�������10g̼����и�Ԫ�ص�����Ϊ![]() ��ʣ������и�Ԫ�ص�����Ϊ4g��
��ʣ������и�Ԫ�ص�����Ϊ4g��


 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ľ̿��ԭ����ͭʵ���Ļ�Ϸ�ĩ�к���ͭ������ͭ��ľ̿�ۣ�ij��ѧʵ��С����ƻ���ͭ�ķ�������:
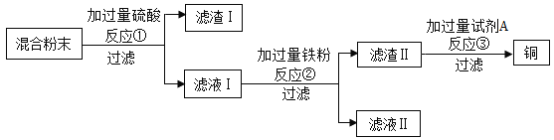
(1)���˲����б����õ��IJ����������ձ�����������___________________�����в�������������__��
(2)��Ӧ�ٵĻ�ѧ����ʽΪ__________________��
(3)��ҺII�е���������Ϊ___________________��
(4)�Լ�A���ѡ������_______________��Һ(�����)��
��H2SO4 ��CuSO4 ��MgSO4
(5)Ϊ������ʵ�鷽�����ɶ�����I�е�______________���л��ա�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʢ����ɽҩ�к��С�ҩ�ûƽ��������أ���ѧʽΪC27H42O3���������й���������˵����ȷ����
A. ������������������
B. ������������Ԫ�ص������������
C. 207g���������к���24g����Ԫ��
D. ������������27��̼ԭ�ӡ�42����ԭ�Ӻ�3����ԭ�ӹ��ɵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬѧ�����о�������̼ʵ������ȡʱ���Ƚ�����������ʵ��:
ʵ�� | A | B | C |
��ȡ�ռ� |
|
|
|
ͬѧ�����о�������̼��ȡʱ���Ƚ�������ʵ��:
(1)��ʵ���ң����Dz�ѡ��ʵ��A��B��ȡ������̼��ԭ��ֱ���___________��__________��ʵ�����Ʊ�����ʱӦ���ǣ���ԭ�����ۡ��ã���________________�������о���ͬѧ��ȷ����ʵ������ȡ������̼��ԭ�����йػ�ѧ����ʽ��________________��
(2) C�м���ƿ��������ð����Ӧ___________________��Ȼ��Ѽ���ƿ������ʵ��̨�ϡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�벻��ʵ��̽����ͨ��ʵ��̽�������ܽ����:���ù��ɣ����Խ����µ�����̽����ʮ�����ͣ��������÷Ŵۼ��չ�ʹ�������ڵĽ��ʯȼ�գ��õ�������̼��̹���ؽ�һ���о�ȷ�Ͻ��ʯ����̼Ԫ����ɣ��������������:
��1�����ʯ����________(����ʡ�����������л��)��
��2�����ϣ����ʯ��________(����ӡ���ԭ�ӡ������ӡ�)����;
��3����ȼ�������������ʯȼ�գ��Ŵۼ��չ��������________��
��4�����ʯȼ�գ��õ�������̼��Ӧ�Ļ���������________��
��5��̹�����о����ʯ��ɵķ�����:��ȡmg���ʯ���������г��ȼ�գ��������ng��CO2,�������̹�����о����ʯ��ɵķ���,��m��n���ݵ�ʽ��ϵ����,̹����ȷ�Ͻ��ʯ��ֻ��̼Ԫ����ɵ����ʵ���ʵ������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ������֮Դ,������ճ������ũҵ�������벻��ˮ,��ͼ��ˮ�ĵ��ʵ��װ��ͼ.
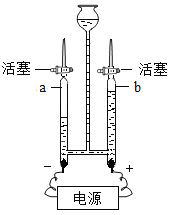
(1)��ʵ����,�������缫������ab��֧�������в���������ֱ���______��______,�����ԼΪ______.
(2)��ʵ��֤����ˮ����__________________________��ɵ�.
(3)������Ϊ,�����ڱ���ˮ��Դ����______(�����).
A ��ҵ��ˮ�ظ�ʹ�� B ����ʹ�û��ʺ�ũҩ
C ��ǿ������ˮ����ϵͳ�Ľ��� D ��������ũ���スˮ
(4)���ˮ�Ļ�ѧ����ʽΪ____________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijƷ�ƿ�Ȫˮ�������װ�ϲ�����������ͼͼ1��ʾ����ش��������⡣
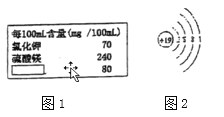
��1������þ����������ӵķ�����________��
��2����֪��ԭ�ӵĽṹʾ��ͼ����ͼͼ2��ʾ����K+����������____________��
��3����֪��ǩ�С������ڵ��������Ȼ��ƣ����Ȼ��ƵĻ�ѧʽ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڻ�ѧ��Ӧ��ֻҪ�ڷ�Ӧǰ����ij��Ԫ�ػ��ϼ۷����ı䣬�÷�Ӧ�Ϳ��Խ�������ԭ��Ӧ�����ݸ����ۣ����з�Ӧ������������ԭ��Ӧ����
A.2KMnO4![]() K2MnO4��MnO2��O2��B.3HClO3��HClO4��2ClO2����H2O
K2MnO4��MnO2��O2��B.3HClO3��HClO4��2ClO2����H2O
C.Na2O2��CO2��H2O��Na2CO3��H2O2D.Fe2(SO4)3��H2SO3��H2O��2FeSO4��2H2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ƽ���л�ѧ����ʽ��
��1��_________C+ _________CO2![]() _________ CO��
_________ CO��
��2��_________Al+_________O2![]() _________Al2O3��
_________Al2O3��
��3��_________CuO+_________C![]() _________Cu+_________CO2����
_________Cu+_________CO2����
��4��_________CH4+_________O2![]() _________CO2+_________H2O��
_________CO2+_________H2O��
��5��_________H2+_________Cl2![]() _________ HCl��
_________ HCl��
��6��_________CuSO4+_________NaOH=____Na2SO4 +_________Cu��OH��2����
��7��_________Mg+_________HCl=_________MgCl2+_________H2����
��8��_________Al��OH��3+_________H2SO4=_________Al2��SO4��3+_________H2O��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com