2005��10��12��--17�գ��ҹ����������š����˺���ɴ���ʤ������ͷ��أ���־���ҹ����պ��켼��������������ˮƽ������ɴ��ڣ��Ա������������Ҫͨ��ʢ��������ﮣ�LiOH���Ĺ��������Գ�ȥ�����Ķ�����̼������ʽ��ʾ��
2LiOH���̣�+CO2������=Li2CO3���̣�+H2O��Һ��
��1���Լ���1g��������������ն�����̼��������
��2�������������أ�KOH��������������ﮣ��Լ���1g���������������ն�����̼��������
��3�����ã�1���ͣ�2�����ý�����Խ���Ϊʲô����ɴ�ѡ��������������ն�����̼������������Ϊ�ѣ�
��4����ÿλ�Աÿ���������Ķ�����̼ƽ����502 L����������λ�Ա�ڽ���һ��Ϊ��5���̫�������Լ���������ɴ���ӦЯ��������﮵�������������ɴ����¶Ⱥ���ѹ�£�������̼������ܶ�Ϊ1.833g/L����
�⣺��1����1gLiOH��������CO
2������Ϊx
2LiOH+CO
2�TLi
2CO
3+H
2O
48 44
1g x
��x=
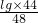
=0.92g
��1g��������������ն�����̼������Ϊ0.92g��
��2����1gKOH�������յ�CO
2������Ϊy
2KOH+CO
2�TK
2CO
3+H
2O
112 44
1g y
��y=
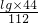
=0.39g
��1g���������������ն�����̼������Ϊ0.39g��
��3��0.92g��0.39g��������LiOH���յ�CO
2��KOH�Ķ࣬�������Լ�������ɴ������أ�
��4��������ɴ�ӦЯ����LiOH������ΪZ

��Z=10001.80g��10kg
��������ɴ���ӦЯ��������﮵�����ԼΪ10kg��
�������Ƚ�������ͬ�����Ķ�����̼������Ҫ�������غ�������﮵�������С��ѡȡ����С���������ն�����̼�ɼ��ٷɴ����أ�
��������λ�Ա��5�������������̼������=502 L��2��5��1.833g/L=9201.66g��

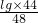 =0.92g
=0.92g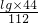 =0.39g
=0.39g


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�