
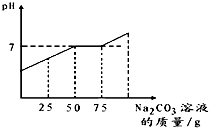
 �����ʵ�顰������̼����ȡ����ҺͰ���㵹�˺��н϶�����Ļ����Һ��Ϊ������Һ��Ⱦ��������ѧ��ȤС����������ʵ�飺ȡ��Һ60g�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ����������CO2����ˮ����Һ����Ե�Ӱ�죩
�����ʵ�顰������̼����ȡ����ҺͰ���㵹�˺��н϶�����Ļ����Һ��Ϊ������Һ��Ⱦ��������ѧ��ȤС����������ʵ�飺ȡ��Һ60g�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ����������CO2����ˮ����Һ����Ե�Ӱ�죩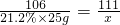
 ��100%=9.25%
��100%=9.25% 

 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?��̨��һģ�������ʵ�顰������̼����ȡ����ҺͰ���㵹�˺��н϶�����Ļ����Һ��Ϊ������Һ��Ⱦ��������ѧ��ȤС����������ʵ�飺ȡ��Һ60g�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ����������CO2����ˮ����Һ����Ե�Ӱ�죩
��2012?��̨��һģ�������ʵ�顰������̼����ȡ����ҺͰ���㵹�˺��н϶�����Ļ����Һ��Ϊ������Һ��Ⱦ��������ѧ��ȤС����������ʵ�飺ȡ��Һ60g�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ����������CO2����ˮ����Һ����Ե�Ӱ�죩�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4�֣������ʵ�顰������̼����ȡ����ҺͰ���㵹�˺��н϶�����Ļ����Һ��Ϊ������Һ��Ⱦ��������ѧ��ȤС����������ʵ�飺ȡ��Һ60g�������м���������������Ϊ21.2����̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ����������CO2����ˮ����Һ����Ե�Ӱ�죩

1.ͨ����ͼ��֪����̼������Һ�����ӵ� gʱ����Һ�е�����ǡ�ô����ꡣ
2.�����Һ���Ȼ��Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱���з�̨���п�һģ��ѧ�Ծ��������棩 ���ͣ�������
��3�֣������ʵ�顰������̼����ȡ����ҺͰ���㵹�˺��н϶�����Ļ����Һ��Ϊ������Һ��Ⱦ��������ѧ��ȤС����������ʵ�飺ȡ��Һ60g�������м���������������Ϊ21.2����̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ����������CO2����ˮ����Һ����Ե�Ӱ�죩

(1)ͨ����ͼ��֪����̼������Һ�����ӵ� gʱ����Һ�е�����ǡ�ô����ꡣ
(2)�����Һ���Ȼ��Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ʵ�顰������̼����ȡ����ҺͰ���㵹�˺��н϶�����Ļ����Һ��Ϊ������Һ��Ⱦ��������ѧ��ȤС����������ʵ�飺ȡ��Һ60g�������м���������������Ϊ21.2����̼������Һ��������ҺpH������̼������Һ��������ϵ����ͼ��ʾ����������CO2����ˮ����Һ����Ե�Ӱ�죩
(1)ͨ����ͼ��֪����̼������Һ�����ӵ� gʱ����Һ�е�����ǡ�ô����ꡣ
 (2)�����Һ���Ȼ��Ƶ�����������
(2)�����Һ���Ȼ��Ƶ�����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com