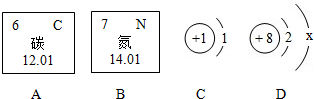
��2013?������һģ��ʯӢ����Ҫ�ɷ�Ϊ�������裬�仯ѧʽΪSiO
2�����Dz�����ҵ���մɹ�ҵ��ԭ�ϣ�ұ��ҵ�����ۼ���
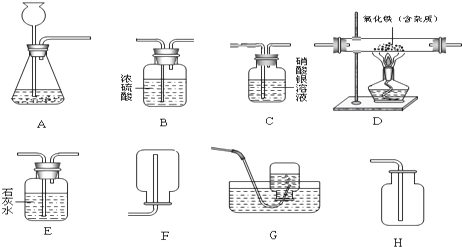
��1��������������
������
������
����ᡱ����������Ρ�������������л����һ�֣���
��2��װ������������Һ���Լ�ƿ�����ò�������ԭ�����ڳ����£�NaOH�벣�����е�SiO
2�����ط�����Ӧ����Na
2SiO
3��H
2O��Na
2SiO
3ʹƿ����ƿ��ճ����һ�𣬸÷�Ӧ�Ļ�ѧ����ʽΪ
2NaOH+SiO2�TNa2SiO3+H2O
2NaOH+SiO2�TNa2SiO3+H2O
��
��3������̫���ܵ�غ͵���оƬ����ȱ�ٵIJ��ϣ������ߴ��������ʾ��ͼ��ͼ��
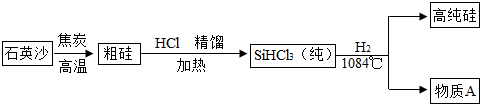
���Ʊ��ֹ�ķ�ӦΪ��SiO
2+2C
Si+2CO������Ӧ��������
�û�
�û�
��Ӧ����ֽ⡱�����ϡ����û��������ֽ⡱֮һ����
�������Ʊ����̱���ﵽ��ˮ����������H
2��ԭSiHCl
3�������O
2�������������
����Σ�գ�����ը
����Σ�գ�����ը
��
������Aͨ��״����Ϊ��ɫ�д̼�����ζ�����壬�ŷŵ������л�������Ⱦ�������Լ���������A���ǣ�
B
B
������ĸ��ţ���
A��Ũ���� B������������Һ C�������� D���Ȼ�����Һ��