
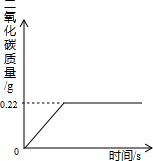
 �ڡ������Ƽ���Ĺ��������У����һ�����ü���NaHCO3�ķ�������ȡ����ģ�ij���������ƵõIJ�ƷNa2CO3��������NaHCO3��Ϊ�˲ⶨ��Ʒ��Na2CO3������������ȡ100g�������ȣ�2NaHCO3=Na2CO3+H2O+CO2����Na2CO3���Ȳ��ֽ⣩����Ӧ����������CO2����������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
�ڡ������Ƽ���Ĺ��������У����һ�����ü���NaHCO3�ķ�������ȡ����ģ�ij���������ƵõIJ�ƷNa2CO3��������NaHCO3��Ϊ�˲ⶨ��Ʒ��Na2CO3������������ȡ100g�������ȣ�2NaHCO3=Na2CO3+H2O+CO2����Na2CO3���Ȳ��ֽ⣩����Ӧ����������CO2����������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ�� Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��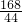 =
=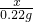
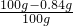 ��100%=99.16%
��100%=99.16%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| �ⶨʱ�� | 5��05 | 5��10 | 5��15 | 5��20 | 5��25 | 5��30 | 5��35 |
| pH | 4.95 | 4.94 | 4.94 | 4.88 | 4.86 | 4.85 | 4.85 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
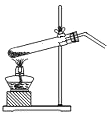

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Si��
��Si�� ����������һ�������մ�ԭ�ϵ���Ҫ�ɷ֣��ܳ��ܸ��£�����������ҵ������ҵ�ȣ���д��������Ļ�ѧʽ________��
����������һ�������մ�ԭ�ϵ���Ҫ�ɷ֣��ܳ��ܸ��£�����������ҵ������ҵ�ȣ���д��������Ļ�ѧʽ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
 A��B���ֹ�����ܽ��������ͼ��ʾ��ij�ձ���ʢ�к�A��B�������ʵ�60��ʱ�ı�����Һ���ұ�����������A��B���壬�����¶ȵĽ��ͣ���
A��B���ֹ�����ܽ��������ͼ��ʾ��ij�ձ���ʢ�к�A��B�������ʵ�60��ʱ�ı�����Һ���ұ�����������A��B���壬�����¶ȵĽ��ͣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | ����ϸ���Ʒ������/g | ��ȡϡ���������/g | ��������������/g |
| 1 | 10.0 | 40.0 | 0.1 |
| 2 | 10.0 | 50.0 | 0.1 |
| 3 | 20.0 | 36.5 | 0.1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com