
ijʵ��С�齫����Ƥ����ԭ����ʯ�ҡ������ʳ�η���ˮ�У���ַ�Ӧ����ˣ��õ�������Һ���Ը���Һ����ɣ��ס���ͬѧ�ֱ����������Ʋ⣺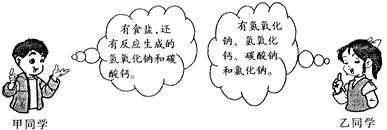
��1������Ϊ������λͬѧ���Ʋⶼ�в��㣬��ͬѧ�Ʋ�����ԭ����___________ ______����ͬѧ�Ʋ�����ԭ���� ��
��2���ҵ��Ʋ��ǣ�����Һ��һ�����ڵ�������_________________��Ϊ��һ��ȷ������Һ���ܵ���ɣ��ס���ͬѧ���������ʵ�����̽����
| ʵ����� ���������Լ��Ļ�ѧʽ�� | ʵ������ | ʵ����� | ����Һ����� |
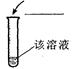 | ���������� | ��Na2CO3 | |
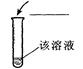 | ������� | ��Ca(OH)2 | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ʵ����� ���������Լ��Ļ�ѧʽ�� | ʵ������ | ʵ����� | ����Һ����� |
 | |||
 | |||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� ���������Լ��Ļ�ѧʽ�� |
ʵ������ | ʵ����� | ����Һ����� |
 |
���������� |
��Na2CO3 |
NaOH��NaCl��Na2CO3 |
 |
�а�ɫ�������� |
��Ca��OH��2 |
NaOH��NaCl��Ca��OH��2 |
�ۺ���������ʵ�� |
������ʵ������������� |
����Na2CO3Ҳ��Ca��OH��2 |
NaOH��NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� ���������Լ��Ļ�ѧʽ�� |
ʵ������ | ʵ����� | ����Һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ʵ����� ���������Լ��Ļ�ѧʽ�� |
ʵ������ | ʵ����� | ����Һ����� |
 HCl HCl |
���������� |
��Na2CO3 |
Na2CO3��NaCl��NaOH Na2CO3��NaCl��NaOH |
 Na2CO3 Na2CO3 |
������� |
��Ca��OH��2 |
Ca��OH��2��NaCl��NaOH Ca��OH��2��NaCl��NaOH |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com