

| OH- | Cl- | SO42- | CO32- | |
| Na+ | ИЬ | ИЬ | ИЬ | ИЬ |
| Ca2+ | Оў | ИЬ | Оў | І» |
| Ba2+ | ИЬ | ИЬ | І» | І» |
| Mg2+ | І» | ИЬ | ИЬ | І» |
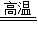 CaO+CO2ЎьЎўCaO+H2OЁTCaЈЁOHЈ©2Ј»
CaO+CO2ЎьЎўCaO+H2OЁTCaЈЁOHЈ©2Ј»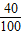 ×100%
×100%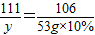
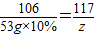
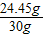 ×100%=81.5%Ј®
×100%=81.5%Ј®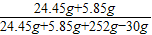 ×100%=10.1%Ј®
×100%=10.1%Ј® CaO+CO2ЎьЈ»CaO+H2OЁTCaЈЁOHЈ©2Ј»
CaO+CO2ЎьЈ»CaO+H2OЁTCaЈЁOHЈ©2Ј»

 МмМмПтЙПТ»ұҫәГҫнПөБРҙр°ё
МмМмПтЙПТ»ұҫәГҫнПөБРҙр°ё РЎС§Йъ10·ЦЦУУҰУГМвПөБРҙр°ё
РЎС§Йъ10·ЦЦУУҰУГМвПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈәФД¶БАнҪв
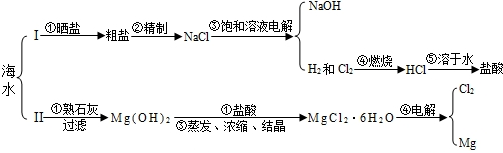
| OH- | Cl- | SO42- | CO32- | |
| Na+ | ИЬ | ИЬ | ИЬ | ИЬ |
| Ca2+ | Оў | ИЬ | Оў | І» |
| Ba2+ | ИЬ | ИЬ | І» | І» |
| Mg2+ | І» | ИЬ | ИЬ | І» |
| ЕдБПұнЈәВИ»ҜДЖЎўКіУГМјЛбёЖЎўөвЛбјШ ҫ»ә¬БҝЈә500g іЙ·ЦұнЈәВИ»ҜДЖЎЭ88% ёЖЈЁТФCaјЖЈ©ЈЁ0.5-1.3Ј©% өвЈЁТФIјЖЈ©ЈЁ20-50Ј©mg/kg |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈәҪвҙрМв
| OH- | Cl- | SO42- | CO32- | |
| Na+ | ИЬ | ИЬ | ИЬ | ИЬ |
| Ca2+ | Оў | ИЬ | Оў | І» |
| Ba2+ | ИЬ | ИЬ | І» | І» |
| Mg2+ | І» | ИЬ | ИЬ | І» |
| ЕдБПұнЈәВИ»ҜДЖЎўКіУГМјЛбёЖЎўөвЛбјШ ҫ»ә¬БҝЈә500g іЙ·ЦұнЈәВИ»ҜДЖЎЭ88% ёЖЈЁТФCaјЖЈ©ЈЁ0.5-1.3Ј©% өвЈЁТФIјЖЈ©ЈЁ20-50Ј©mg/kg |
Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com