



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�п����� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
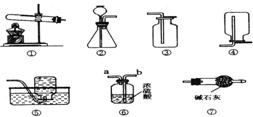
��ˮ��һ��ȡ֮��������֮���ߵ���Դ���⡣��Χ�ƿ�ѧ���ú�ˮ��Դ����д�ո�
���ú�ˮ��ɹ�Ρ��ķ����õ��Ĵ����У����Ȼ����⣬�������Ȼ�þ���Ȼ��ơ������Ƶ����ʡ����˴����ᴿ�IJ���ʵ�鷽�����£�
�ټ�������������Һ��Ŀ����_____________________________________��
�ڹ��˺�õ��ij����ɷ��У�������þ��̼��ơ�___________________��
�������ʵ�飬��ͨ�������ɼ�������ȷ������������Һ��Ʒ�м���ϡ���ᣬ��ǡ����ȫ��Ӧ��_____________________________________________________________��
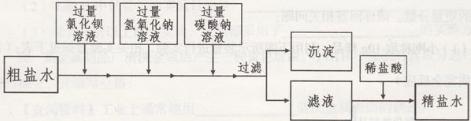
����ͼ�Ǻ�ˮ���Ƽ
�ٽ�����ͨ�뱥��ʳ��ˮ�У����Ƴɱ��Ͱ���ˮ(������������ˮ)������ˮ��ʳ��ˮ���������ն�����̼��ԭ���ǣ�______________________________________��
����д���Ȼ����Һ����ʯ�ҷ�Ӧ�Ļ�ѧ����ʽ��_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���������װ�ã��ش����⣺

 |
��1��д����Ţٺ͢ڵ��������� ��
��2��װ��B�������ٵ��¶˹ܿ�Ҫ����Һ���£���Ϊ�� ��
��3��ʵ���Ҽ��Ȱ���ɫ������ȡO2�ķ�Ӧ���ű���ʽΪ ��������ˮ���ռ����壬���۲쵽_____ ___ʱ�ſ�ʼ�ռ���ֹͣʵ��ʱ��Ӧ��_____ _____ _������_______________ ____________��
��4������װ��C��D��K�ռ�O2��Ҫʹ�ռ�O2�����Ҵ�������Ӧѡ װ�á�������ˮ���ռ�����O2���������ܵ�ԭ���ǣ���������_____________ ____________��_________________ ___________��
���������ſ������ռ����������������_________ ____________ _________��
ʵ������з���ˮ���е�ˮ�����dz�Ϻ�ɫ�����ܵIJ���ԭ��_____ ___________ ��
��5���ù���������Һ�����������ȡO2��װ��G��Ȼ������㣬��Ҫ�õ�ƽ�ȵ���������H��I��J��ѡȡ�� ��ȡ��G�еĵ��������ԴﵽĿ�ġ���ѡ��H����ƿ��װ�ķ���װ�ã�����Ӧֹͣʱ������ƿ�л�������O2��Ϊ����O2���ڲ���жװ�õ�ǰ���£����� ��
��ʵ�������ͬѧ�����ռ����������Ԥ�Ƶ��ٺܶ࣬��˼������⡣����Ϊ���ܵ�ԭ���ǣ�___________ ___ _________��
��6����������غͶ������̵Ļ������ȡO2��Ĺ���������ٶ�����ȫ��Ӧ����ͨ�������IJ�ʵ������ɻ��ն������̡���ȷ�������Ⱥ�˳���� ����дѡ����ţ���
a����� b���ܽ� c������ d��ϴ��
��7��ʵ��������Fװ���Ʊ����������Ƚ�п�������п���֮�ϣ�Ȼ�����һ�˼���ϡ���ᣬװ��F�����װ��B�Ʊ����������е��ŵ��� ������Lװ���ռ�������������� ��ͨ�루�a����b������ͬ������Ҫ��Lװ���е�������ˮ�ų�����ˮ�� ��ͨ�롣
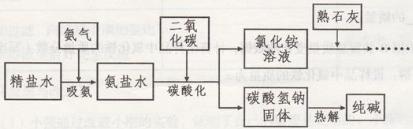
 ��8���������ϣ�ʵ���ҳ��ü����Ȼ�泥�NH4Cl������ʯ��[Ca(OH)2]���ֹ�������
��8���������ϣ�ʵ���ҳ��ü����Ȼ�泥�NH4Cl������ʯ��[Ca(OH)2]���ֹ�������
�ķ�����ȡ������NH3����ͬʱ�������Ȼ��ƣ�CaCl2����ˮ����÷�Ӧ�ķ��ű���ʽΪ
�����⣬�����д̼�����ζ�Ҽ�������ˮ������
ʵ�����ﰱ������ȡװ����_________��С���������ͼ��ʾ��һ��ʵ�飺����������
һ֧�Թܵ�����һ��װ������ˮ���ձ��С�һ��ʱ����ῴ���� ��
��9��ij��ѧС����ѧϰ����ȡO2�����Ƿ�����ʵ��������5% H2O2��Һ������O2���ô����ǵ�ľ�����飬ľ�����Ѹ�ȼ��ͬѧ�Ǿ�������۲죬���������ԭ���� ��
Ϊ����֤�˲����Ƿ���ȷ����ȤС���ͬѧ�����������ۣ��ƶ���ʵ�鷽����������ʵ�顣
��һ��ͬѧ��ȡ����H2O2��ҺŨ�ȵķ�������10%��H2O2��Һ���ȣ�
 һ��ʱ����ô����ǵ�ľ�����飬ľ����ȼ��
һ��ʱ����ô����ǵ�ľ�����飬ľ����ȼ��
�ڶ���ͬѧ��5%��H2O2��Һ�м���2����������������Һ������
һ��ʱ����ô�����ľ�����飬ľ����ȼ��
������ͬѧ��ʵ��װ���Ͻ����˸Ľ���Ч���ܺã���ͼ����
��ʵ������ϣ�ͬѧ�Ǿ����������ۣ��ó���һЩ���ۣ�
���ɵ�һ��ʵ��ɵó�Ӱ��H2O2�ֽ����O2���ټ�����������֮һ�� ��
���ɵڶ���ʵ��ɷ����ó�����������������ÿ����� ��
�۵�����ʵ����֤��ʵ��ǰͬѧ�Dz������ȷ�ԡ�����Ũ����������� ��
��С��ͬѧ����۲��˵�����ͬѧ��ʵ��װ�ã������һ�ּĸĽ�װ�ã����ֳ�ʵ�飬Ч���ܺã�����˵�����Ľ��ĵط��� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com