


 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д� ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ʾ������Ϊl��ԭ�ӣ���
����ʾ������Ϊl��ԭ�ӣ��� ��ʾ������Ϊ8��ԭ�ӣ���ش��������⣺
��ʾ������Ϊ8��ԭ�ӣ���ش��������⣺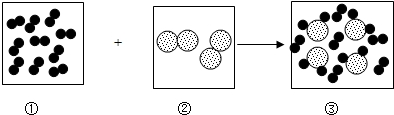
 ��
�� ��������
�������� ��������Ϊ
��������Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�Ǵӷ��ӡ�ԭ�ӵ��ӽ���ʶ���ʵģ��밴Ҫ��ش����⣺
��1���ڻ�ѧ��Ӧǰ�϶�������ǣ� �� 
A. ԭ�ӵ����� B. ԭ�ӵ���Ŀ
C. ���ӵ����� D. ���ӵ���Ŀ
��2���û�ѧ������д����1��ͭԭ�� ��3��ˮ���� ��
��3������ͼʾ�ش�A��B��C��D��ʾ4������,����ʾ��ͼ������ʾ��A��B��һ�������·�Ӧ����C��D��
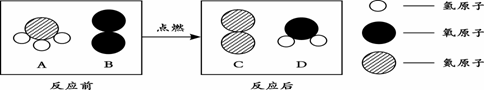
����ͼʾ�ش�A��B��C��D��ʾ4������,����ʾ��ͼ���±���A��B��
��һ��A�����к� ��ԭ�ӣ�
��������������� ��
�۲μӷ�Ӧ��A��B���������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com