
| ��һ�� | �ڶ��� | ������ | |
| ʣ����������/g | 16.75 | 13.50 | 12.40 |
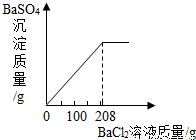
 ×100%����ã���2��Ҫ��ڶ���ʵ���зų�������������ӱ����п�֪��һ�κ͵ڶ���֮��������˵��������Ǽ��ٵ�п�����ᷴӦ���ɵ������ˣ��ɴ˸��ݻ�ѧ����ʽ��֪��Ӧ�����������������������3�����ݡ����ó�����������BaCl2��Һ�����Ĺ�ϵͼ��ʾ�����õ�BaCl2��Һ������Ϊ200�ˣ���Ϊ�μ�BaCl2��ҺҪ���ɲ�����ˮ��BaSO4��ɫ������ʵ�����Ǻ�ϡ�����е���������ӽ�ϣ�����ֻҪ���������������Ϳ�������Ȼ�����������
×100%����ã���2��Ҫ��ڶ���ʵ���зų�������������ӱ����п�֪��һ�κ͵ڶ���֮��������˵��������Ǽ��ٵ�п�����ᷴӦ���ɵ������ˣ��ɴ˸��ݻ�ѧ����ʽ��֪��Ӧ�����������������������3�����ݡ����ó�����������BaCl2��Һ�����Ĺ�ϵͼ��ʾ�����õ�BaCl2��Һ������Ϊ200�ˣ���Ϊ�μ�BaCl2��ҺҪ���ɲ�����ˮ��BaSO4��ɫ������ʵ�����Ǻ�ϡ�����е���������ӽ�ϣ�����ֻҪ���������������Ϳ�������Ȼ����������� ×100%=
×100%=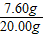 ×100%=38%
×100%=38% =
=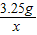
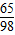 =
= ��ã�Y=4.9g
��ã�Y=4.9g 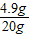 ×100%=24.5%
×100%=24.5%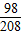 =
= ��Z=31.2g��
��Z=31.2g��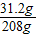 ×100%=15%
×100%=15%

 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2010�걱���к������п���ѧ��ģ�Ծ��������棩 ���ͣ������
| ά����C����ɫ�� ��ѧʽ��C6H8O6 100mg/Ƭ Vc����10% ÿ��2Ƭ ÿƿ60Ƭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���к������п���ѧ��ģ�Ծ��������棩 ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���к������п���ѧ��ģ�Ծ��������棩 ���ͣ������
| ʵ �� װ �� ͼ |  | 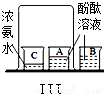 |  |
| �� �� �� �� �� | ʵ��I������ɫʯ����Һ��ͨ��CO2����ʵ��ʹ��ɫʯ����Һ��������Ϊ____�� ʵ��II�ǽ�ʵ��I�����õĻ��Һ���ȣ�ʵ������Ϊ_____��������Ӧ�Ļ�ѧ����ʽΪ��__________________�� | ʵ��III��������___________________________����ʵ��˵���ˣ�����ţ�___________________�� �ٰ����Ӳ����˶� �ڷ�̪���Ӳ��˶� �۰�ˮ�ʼ��� �ܰ�ˮ�ӷ� | ʵ��IV��ʵ��Vװ���У�����ɲⶨ��������������������ǣ�����ţ�__________������ȼ�յĻ�ѧ����ʽΪ_________________�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���к������п���ѧ��ģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com