




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶ȣ��棩 | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�� ��g�� | NaOH | 31 | 91 | 111 | 129 | 313 | 336 |
| Ca��OH��2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 | |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶ȣ��棩 | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�� ��g�� |
NaOH | 31 | 91 | 111 | 129 | 313 | 336 |
| Ca��OH��2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 | |
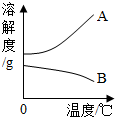

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п���ѧ����������23��ͼ�Ļ�ѧ���� ���ͣ���Ϣ������
��2011��㶫��21�⣩�±���NaOH��Ca(OH)2���ܽ�����ݣ���ش��������⡣


��1���ӱ������ݿ��Ի�õ���Ϣ��___________��дһ������
��2����80��ʱNaOH�ı�����Һ������20�棬���Կ�����������_______������20����Ca(OH)2�ı�����Һ������Һ���������м���һ����CaO��õ�����Һ������Һ������ʱ��Һ����������������______�ף��������������
��3��ij��ȤС��Բ��ֱ��ʵ��������ƹ�������ᴿ����������²������̣���ش�


������ٷ�Ӧ�Ļ�ѧ����ʽΪ________���������Ca(OH)2��Ŀ����______________��
������ҺB�е�������__________��_________��д��ѧʽ����������������ľ�������Ǽ���Ũ����_______�����ˡ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com