
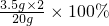 =35%���ʴ�Ϊ��35%��
=35%���ʴ�Ϊ��35%��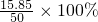 =31.7%��
=31.7%��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ���������������꼶��ѧ�����У�һģ�����Ի�ѧ�Ծ����������� ���ͣ������
����������Ƽ��������ȶ��벻����ѧ
��1����Դ��������������������ᷢչ������ء�
��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ� ��
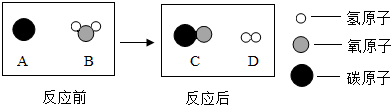
��Ϊ������Ⱦ�����ú�������ʣ��ɽ���ת��Ϊ��ȼ������ˮú�����˹��̿���Ϊ��̼��ˮ�ķ�Ӧ������ʾ��ͼ������ʾ��
�÷�Ӧ�Ļ�����Ӧ����Ϊ ���÷�Ӧ������ķ��Ӹ�����Ϊ ��
��ˮú���ڲ�ͬ���������£����Ժϳɲ�ͬ���ʣ�����Ҫ�л���״���CH3OH����
д���÷�Ӧ�Ļ�ѧ����ʽ ��������Ϊ������ˮú���ڴ��������²��ܺϳ����ء�CO(NH2)2���������� ��
��2�����ࡢ��֬�������ʶ�����������Ӫ�����ʣ�������ʳ�ǽ����Ļ�����֤��
��ʳ��ijɷ���Ҫ�е����ʡ� ����֬��ά���ء����κ�ˮ�������࣬ͨ
����ΪӪ���ء�����ʳƷ�и��������ʵ��� ����д��ţ���
A������ B������ C�������� D���� E��ţ��
�ڻƹ��и���ά����C��ά����C��pH��5�͵��µĻ����н��ȶ���Ϊ����ά����C����ʧ��ʳ�ûƹϵ���ѷ����� ��
�۴����еĵ��۷ֽ�����������պ��������ϸ���ĺ������ã�����������̼���ͷ�������ά�����������д����������һ�仯�Ļ�ѧ����ʽ ��
��3�����������������������Ҫ���ʻ�������������ܶ࣬ͨ���ɷ�Ϊ�������ϡ����ǽ������ϡ��߷��Ӻϳɲ��ϼ����ϲ��ϡ�
��������������ͭ���������ˡ����������������ǵ� ��������ʹ�������������������еĹ�ϵ�����������Լ����������ֽ�����̽�����ǵĽ������˳���ܴﵽĿ���� ������ţ���
A����������Һ B������������Һ C������ͭ��Һ
�������йغϽ����ʵ�˵����ȷ���� ������ĸ����
A���Ͻ���۵�һ������ijɷֽ����� B���Ͻ��Ӳ��һ������ijɷֽ�����
C����ɺϽ��Ԫ��������ͬ���Ͻ�����ܾ�һ����ͬ
D���Ͻ�����ɷֽ�����ȣ�����������������������ѧ���е����
�۸����� ������Ͻ𡱻����Ͻ𡱣���
�����жԽ�����Ʒ��ȡ�ķ�����������ȷ���� ������ţ���
A���ڵ��ߵ��������һ�����ϲ� B�������г���Ȧ�϶���һ�������
C���ں��ֵ���������Ϻ���ͭ
�ݺϳ����ϡ��ϳ��� �dz�˵������ϳɲ��ϡ��������ںϳ����� ����ĸ����
A��������̥ B������ C��������ϩ��Ʒ D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����������Ƽ��������ȶ��벻����ѧ
��1����Դ��������������������ᷢչ������ء�
��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ� ��
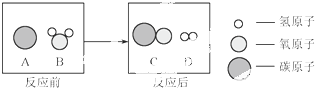
��Ϊ������Ⱦ�����ú�������ʣ��ɽ���ת��Ϊ��ȼ������ˮú�����˹��̿���Ϊ��̼��ˮ�ķ�Ӧ������ʾ��ͼ������ʾ��

�÷�Ӧ�Ļ�����Ӧ����Ϊ ���÷�Ӧ������ķ��Ӹ�����Ϊ ��
��ˮú���ڲ�ͬ���������£����Ժϳɲ�ͬ���ʣ�����Ҫ�л���״���CH3OH����
д���÷�Ӧ�Ļ�ѧ����ʽ ��������Ϊ������ˮú���ڴ��������²��ܺϳ����ء�CO(NH2)2���������� ��
��2�����ࡢ��֬�������ʶ�����������Ӫ�����ʣ�������ʳ�ǽ����Ļ�����֤��
��ʳ��ijɷ���Ҫ�е����ʡ� ����֬��ά���ء����κ�ˮ�������࣬ͨ
����ΪӪ���ء�����ʳƷ�и��������ʵ��� ����д��ţ���
A������ B������ C�������� D���� E��ţ��
�ڻƹ��и���ά����C��ά����C��pH��5�͵��µĻ����н��ȶ���Ϊ����ά����C����ʧ��ʳ�ûƹϵ���ѷ����� ��
�۴����еĵ��۷ֽ�����������պ��������ϸ���ĺ������ã�����������̼���ͷ�������ά�����������д����������һ�仯�Ļ�ѧ����ʽ ��
��3�����������������������Ҫ���ʻ�������������ܶ࣬ͨ���ɷ�Ϊ�������ϡ����ǽ������ϡ��߷��Ӻϳɲ��ϼ����ϲ��ϡ�
��������������ͭ�������� �ˡ����������������ǵ� ��������ʹ�������������������еĹ�ϵ�����������Լ����������ֽ�����̽�����ǵĽ������˳���ܴﵽĿ���� ������ţ���
�ˡ����������������ǵ� ��������ʹ�������������������еĹ�ϵ�����������Լ����������ֽ�����̽�����ǵĽ������˳���ܴﵽĿ���� ������ţ���
A����������Һ B������������Һ C������ͭ��Һ
�������йغϽ����ʵ�˵����ȷ���� ������ĸ����
A���Ͻ���۵�һ������ijɷֽ����� B���Ͻ��Ӳ��һ������ijɷֽ�����
C����ɺϽ��Ԫ��������ͬ���Ͻ�����ܾ�һ����ͬ
D���Ͻ�����ɷֽ�����ȣ�����������������������ѧ���е����
�۸����� ������Ͻ𡱻����Ͻ𡱣���
�����жԽ�����Ʒ��ȡ�ķ�����������ȷ���� ������ţ���
A���ڵ��ߵ��������һ�����ϲ� B�������г���Ȧ�϶���һ�������
C���ں��ֵ���������Ϻ���ͭ
�ݺϳ����ϡ��ϳ��� �dz�˵������ϳɲ��ϡ��������ںϳ����� ����ĸ����
A��������̥ B������ C��������ϩ��Ʒ D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ���������������꼶���£����л�ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com