
�������ʶ�������ˮ�ʵľ�����ɱ��������
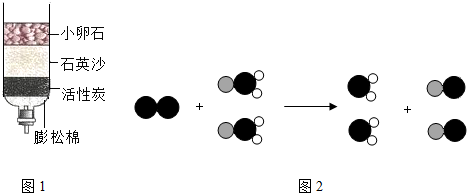
��1�������ͻ���̼����ˮ�м������������ܽ⣬����һ��ʱ����� ����������ƣ����Ӷ���ȥ����С�������������õij���Һ�м������̿��������
��ȥˮ�е���ɫ����ζ��
��2������(��ѧʽO3)����ǿ�����ԣ���������Ӿ�ء�������ˮ����ˮ��ɱ�����������������������Ԫ����ͬ����ѧ���ʲ�ͬ��ԭ���� ��
��3��Ư�ۣ�������ˮ��ɱ������������Ч�ɷ��Ǵ������[��ѧʽΪCa(ClO)2]��������ƿɷ������·�Ӧ��Ca(ClO)2 + X + H2O��CaCO3��+2HClO����X�Ļ�ѧʽΪ ��
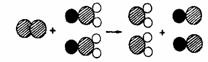
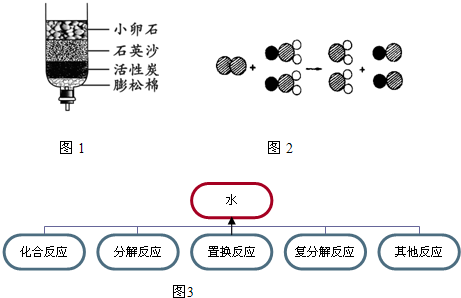
��4��ClO2������ˮ������ClO2��������ȡClO2�ķ�Ӧ���۹�����ͼ��ʾ������![]() ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�![]() ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�![]() ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ���
��д����Ӧ�Ļ�ѧ����ʽ ��
 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
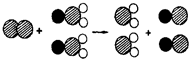 25���������ʶ�������ˮ�ʵľ�����ɱ��������
25���������ʶ�������ˮ�ʵľ�����ɱ�������� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ���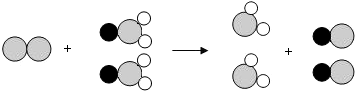 ��д����Ӧ�Ļ�ѧ����ʽ
��д����Ӧ�Ļ�ѧ����ʽ ��ʾ��
��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ� ��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
��ʾ��ԭ�ӣ�����д����Ӧ�Ļ�ѧ����ʽ
| ||
| ||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com