
| ʵ�鲽�� | ��� |
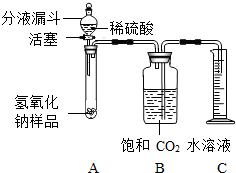
1����ͼ���Ӻ�װ�ã� |
1��д���ٺ͢ڵ��������� �� �Թ� �Թ� �� ��Ͳ ��Ͳ |
| 2������ƽȷ��ȡ����������Ʒ2g�������������У���B�м���ƿ�ڵ��뱥�Ͷ�����̼ˮ��Һ��ƿ���� | 2��B�м���ƿʢװ�ı��Ͷ�����̼ˮ��Һ������ˮ���棬�������� ���������̼�ܽ���ˮ�����ģ���ɲⶨ���ƫ�� ���������̼�ܽ���ˮ�����ģ���ɲⶨ���ƫ�� |
| 3�����Һ©���е���ϡ���ᣬ��������ϡ������������������������رջ�������Ӧ���������ռ������Ͷ�����̼ˮ��Һ220mL�� | 3�����ж�ʵ�鲽��3�е����ϡ�����ѹ����ı�־�� ϡ���������Ʒ��û�����ݲ��� ϡ���������Ʒ��û�����ݲ��� ��д���������ݵĻ�ѧ��Ӧ����ʽ��Na2CO3+H2SO4�TNa2SO4+H2O+CO2�� Na2CO3+H2SO4�TNa2SO4+H2O+CO2�� |
| 4����������Ʒ��̼���Ƶ���������Ϊ53.0%�� | 4������ƿ���ռ���������̼�������� 0.44 0.44 g������27�棬101kPaʱ������̼���ܶ�ԼΪ2g/L��������̼����������ų��ı��Ͷ�����̼ˮ��Һ��������� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
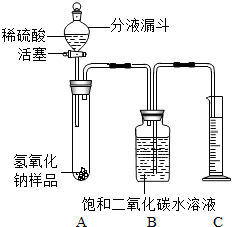 ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ��������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ��������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����һ�� ȡ��������ˮ���μ� �������Ȼ�����Һ ȡ��������ˮ���μ� �������Ȼ�����Һ |
�а�ɫ�������� �а�ɫ�������� |
�������Ʋ��ֱ��� |
| ������� ���ã�������Һ�еμ� ��̪ ���ã�������Һ�еμ� ��̪ |
��� ��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com