
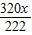
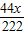
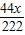 =32g
=32g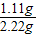 ×100%=50%
×100%=50%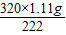 =1.6g
=1.6g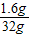 ×100%=5%
×100%=5%


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ۺ�Ӧ�ô������¿α�9�꼶��ѧ�� ���ͣ�038
ij��ȤС��ӹ�ͭ���Ϲ���һЩͭ����м��Ϊ�˲ⶨ��Щ��м��
Cu2(OH)2CO3�ĺ�������С���ȡ��2.22g��м�����ձ��У�Ȼ������ϡ���ᣬ�ȣ��������з�Ӧ�� Cu2(OH)2CO3��2H2SO4��2CuSO4��CO2����3H2O��ǡ����ȫ��Ӧʱ����ȥϡ����
31.11g���õ������͵�����ͭ��Һ32g��������м�е��������ʼȲ�����ˮҲ�����й����ʷ�Ӧ���Լ��㣺(1)��ʽ̼��ͭ����Է���������
(2)��м�м�ʽ̼��ͭ������������
(3)���õ�������ͭ��Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���人 ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com