


 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
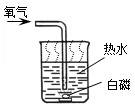
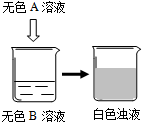
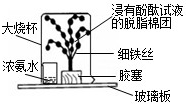 ��2009?���Ƹۣ�2009�괺�����������ϣ���ǫ��ħ�����ݼ���������ǿ�ҵĺ����ģ�����ȫ����Χ��������ħ�����ݵ��ȳ�����ѧУ�Ƽ��ڻ�У�һλ���꼶ͬѧ�����꼶ͬѧ������һ�黯ѧСħ��������������ѧ��ѧ֪ʶ�ҿ�����֮�գ�
��2009?���Ƹۣ�2009�괺�����������ϣ���ǫ��ħ�����ݼ���������ǿ�ҵĺ����ģ�����ȫ����Χ��������ħ�����ݵ��ȳ�����ѧУ�Ƽ��ڻ�У�һλ���꼶ͬѧ�����꼶ͬѧ������һ�黯ѧСħ��������������ѧ��ѧ֪ʶ�ҿ�����֮�գ�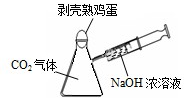
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2009?���Ƹۣ���ȥ���������л��е����ʣ���ѡ�õ��Լ���������������ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2009?���Ƹۣ� NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ��ʾ�������ͼ����й���Ϣ�ش��������⣺
��2009?���Ƹۣ� NaNO2���������ƣ���NaCl������M �������ᾧˮ�����ܽ��������ͼ��ʾ�������ͼ����й���Ϣ�ش��������⣺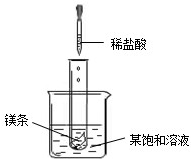
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com