
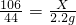 ��ã�X=5.3g
��ã�X=5.3g ��100%=94.6%
��100%=94.6%

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�������ʡ�������п���ѧ���� ���ͣ�058
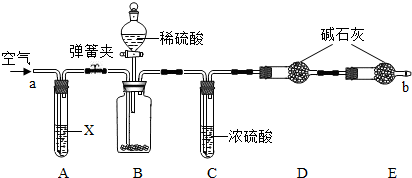
�����Ƽ���õĴ����г����������Ȼ��ƣ�������ͼ��ʾװ�����ⶨ������Ʒ��̼���Ƶ���������(����̨�����еȹ̶�װ������ȥ)��

ʵ�鲽�����£�
�ٰ�ͼ����װ�ã�����������ԣ�
��ȷ�Ƶ�ʢ�м�ʯ��(�����������ƺ������ƵĻ����)�ĸ����D������Ϊ83.4 g��
��ȷ�Ƶ�6.0 g������Ʒ����װ��B�Ĺ��ƿ�У�
�ܴ�װ��B�ķ�Һ©����������������ϡ���ᣬ�����ٲ�������Ϊֹ��
�ݴ��ɼУ����Թ�A�л���������������ӣ�Ȼ��Ƶø����D��������Ϊ85.6 g��
�Իش�
(1)���������Ŀ����________��װ��A���Լ�X������ѡ��________��
(2)��û��Cװ�ã���ᵼ�²ⶨ���________(���ƫ��ƫС��)��
(3)Eװ�õ�������________��
(4)�����ʵ���в�õ��й����ݣ����㴿����Ʒ��̼���Ƶ�����������(Ҫ��д��������̣��������1λС��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ�������������������ѧ���������� ���ͣ�������
�����ƼΪ���ҵ�ķ�չ�����˽ܳ��Ĺ��ף��ô˷����ƵõĹ�ҵ�����Ʒ�лẬ��һ�������Ȼ��ƣ�Ϊ�ⶨһ���ú����Ƽ�����Ĺ�ҵ�����Ʒ��̼���ƵĴ��ȣ���ȡ26.5g�ù�ҵ������Ʒ������100gij��������������ϡ���ᣬǡ����ȫ��Ӧ���õ�117.7g��������Һ����ش��������⣺
��1������ϡ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ�� ����
��2�������Ʒ��̼����������x���ı���ʽΪ������
��3����Ʒ��̼���ƵĴ���Ϊ�� ����
��4������Ӧ�����Һ�м���54.5gˮ����������Һ�����ʺ��ܼ�����������Ϊ�� ����
��5����ҵ�ϳ���̼���ƺ��������Ʒ�Ӧ����ȡ�������ƣ���Ҫ�����ַ����Ƶ�20t����������80%�Ĺ�ҵ�ռ��Ҫ�����ú����Ƽ��õĹ�ҵ�����Ʒ���������� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ�������������������ѧ�������棩 ���ͣ�������
�����ƼΪ���ҵ�ķ�չ�����˽ܳ��Ĺ��ף��ô˷����ƵõĹ�ҵ�����Ʒ�лẬ��һ�������Ȼ��ƣ�Ϊ�ⶨһ���ú����Ƽ�����Ĺ�ҵ�����Ʒ��̼���ƵĴ��ȣ���ȡ26.5g�ù�ҵ������Ʒ������100gij��������������ϡ���ᣬǡ����ȫ��Ӧ���õ�117.7g��������Һ����ش��������⣺
��1������ϡ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ�� ����
��2�������Ʒ��̼����������x���ı���ʽΪ������
��3����Ʒ��̼���ƵĴ���Ϊ�� ����
��4������Ӧ�����Һ�м���54.5gˮ����������Һ�����ʺ��ܼ�����������Ϊ�� ����
��5����ҵ�ϳ���̼���ƺ��������Ʒ�Ӧ����ȡ�������ƣ���Ҫ�����ַ����Ƶ�20t����������80%�Ĺ�ҵ�ռ��Ҫ�����ú����Ƽ��õĹ�ҵ�����Ʒ���������� ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com