һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH
4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��
��1����ҵ������Ȼ����ˮ�����ڸ��������·�����Ӧ���õ�CO��H
2���÷�Ӧ�Ļ�ѧ����ʽΪ
���õ���CO��H
2�Ļ��������Ϊ�ϳ������ϳ����ڹ�ҵ�Ͽ����ںϳ�һϵ�л���ԭ�Ϻ�����ȼ�ϣ���������ұ��ijЩ������
��2��ij��ѧ��ȤС���ͬѧ��ʵ����ģ���˺ϳ������Ʊ��������ʵ����֤�ϳ����Ļ�ԭ�ԣ�
I���ü����ˮ�����ڸ��������·�Ӧ�õ��ϳ��������� ��1������д�Ļ�ѧ����ʽ���ϳ�����CO��H
2��������Ϊ
14��3
14��3
��
���úϳ�����ԭ��������ͭ��ʵ��װ����ͼ��ʾ��
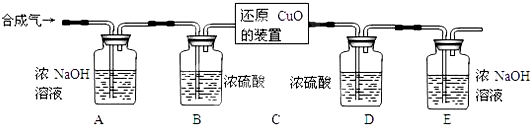
[���ʵ�鲽��]
�����Ӻ�װ�ò���������ԣ�
��װ��ҩƷ��ͨ��һ��ʱ��ϳ�����
�۷ֱ����װ��D��E��������
�ܻ���ͨ��ϳ���������װ��C��ʹ���ַ�Ӧ��
��ֹͣ���ȣ�����ͨ��ϳ������ָ������£�
���ٴηֱ����װ��D��E��������
[����ʵ�����]��������ͬѧ����Ϊ��װ��E��Ӧ����һ���ƾ��ƣ�����ʵ�鲽��
��
��
����ʵ�鲽�����ţ��е�ȼ����ȼǰӦ�������鴿����
[����ʵ��]��С���ͬѧ�������������ʵ�飬����¼��ʵ����������ݣ�
��װ��C�г���
��
��
ɫ���ʣ�
�ڳ��������������ʾ��
|
װ��D������ |
װ��E������ |
| ��Ӧǰ |
292.4g |
198.2g |
| ��Ӧ�� |
296.0g |
201.5g |
[������������ݴ���]
�ټ�ͬѧ����װ��C�е�������Ϊ�Ǻϳ����е�CO��H
2��ԭ��CuO��
����ͬѧͨ�����ϱ����ݵķ������Ʋ����CO��H
2���⣬�Ƶõĺϳ����п��ܻ���δ��Ӧ��CH
4����CH
4Ҳ��ԭ��CuO����ͨ������˵�������Ʋ����ݣ�
��ʵ����������̼����Ԫ�ص���������һ����̼��������ԭ����ͭ��̼����Ԫ�ص������Ȳ���
��ʵ����������̼����Ԫ�ص���������һ����̼��������ԭ����ͭ��̼����Ԫ�ص������Ȳ���
[��չ̽��]��С��ͬѧ����CH
4�Ƿ�����ܹ���ԭ����ͭ��������ɲ�����̽����
�������ϣ�������л�ԭ�ԣ����Ի�ԭ����ͭ������CO
2��H
2O����ɫ����ˮ����ͭ��ˮ������ɫ��
ʵ����ƣ���С��ͬѧ���ô����ļ������������װ�ý���ʵ�飮
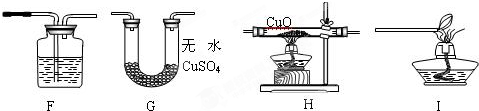
��ͼ��װ��F��ʢ�ŵ��Լ�������
�����ʯ��ˮ
�����ʯ��ˮ
��
��ͼ��װ�õ���ȷ����˳����
HGFI
HGFI
������ĸ����ÿ��װ������һ�Σ���






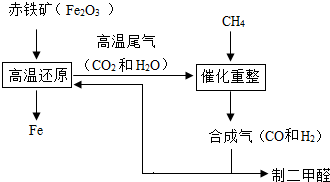 һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��CO�Ǵ�ú��������ϳ����õ��ģ�ú��������Ҫ��Ӧ�У�
һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��CO�Ǵ�ú��������ϳ����õ��ģ�ú��������Ҫ��Ӧ�У�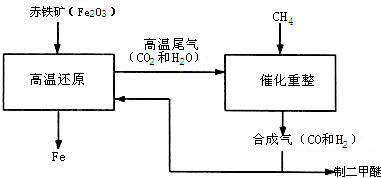
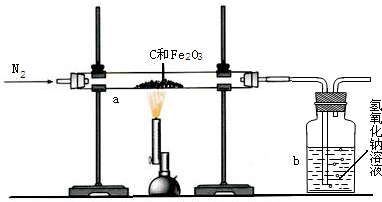
 ��2011?������һģ��һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ����ҵ�ϳ�����Ȼ����ˮ�����������̼�����´���Ӧ�õ�CO��H2���õ���CO��H2�Ļ��������Ϊ�ϳ�������ͼΪ���úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̣�
��2011?������һģ��һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ����ҵ�ϳ�����Ȼ����ˮ�����������̼�����´���Ӧ�õ�CO��H2���õ���CO��H2�Ļ��������Ϊ�ϳ�������ͼΪ���úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̣� ��1�����ø�¯β���е�ˮ���������������õ��ϳ�������ѧ����ʽΪ
��1�����ø�¯β���е�ˮ���������������õ��ϳ�������ѧ����ʽΪ