
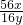 =
=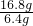 ��������
��������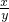 =
=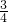 �����Ը�����������Ļ�ѧʽ��Fe3O4��
�����Ը�����������Ļ�ѧʽ��Fe3O4��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д� �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�������������п���ѧ��ģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
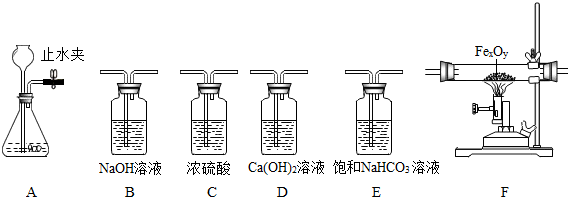
��ѧ��٤����˵��һ������������ӹ۲���ʵ���е�������������ʦ�ṩ��һЩʵ��װ�ã������Ҫ��ش��������⣺

(1) �ô���ʯ��ϡ���ᷴӦ (ϡ����ӷ�����HCl������ñ���NaHCO3��Һ����) ��ȡ���ռ����﴿
�Ķ�����̼���塣
�� �ر�Aװ���е�ֹˮ�кӳ���©������ƿ��ע��һ������ˮ����ֹ����ͼ��ʾ�� ��Aװ���Ƿ�©��? (�©����������©��������ȷ����֮һ)��
�� ʵ������ȡ������̼�Ļ�ѧ����ʽ��
�� ��ѡ����������˳�� �� �����ռ�װ�� (��д���������ĸ)
(2) ijͬѧ���ú��ж�����̼��ˮ�������ʵ�CO�ⶨһ������������ (FeXOY) ����ɣ����������������������Ϊ23.2g�����ʵ�鷽�������������Ǵ������ң�ѡ���������B��C��F��D˳�����ӣ�Ȼ�����ʵ�� (�����йط�Ӧ����Ӧ��ȫ)��
��װ��B�������� ��
�� Fװ���е�FeXOY ȫ������ԭ����ʣ����������Ϊ16.8g��������������Ļ�ѧʽ
Ϊ
�� ��������������ʱ�����û��Bװ�ã��ⶨ�������Ԫ������Ԫ�ص�������ֵ�� (�ƫ��ƫС�����������䡱֮һ)
�� ����װ������һ�����ԵIJ���֮����Ӧ��װ��D�����ȼ�ŵľƾ��ƴ���β������������Ŀ��
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com