
(5��)������ұ��������������һ����Ҫ��־��ͼ1��ʵ����ģ��������װ��ͼ��



ͼһ ͼ�� ͼ��
(1)д��ͼ1��A�������Ļ�ѧ��Ӧ����ʽ�� ��
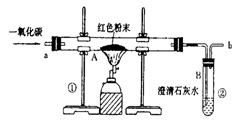
(2)ʵ�������ͨ��CO�������ɫ��ĩ�����Ĺ�ϵ��ͼ2��ʾ������ͼʾ������˵������ȷ���� ��������ţ�
��a���ʾ��ʼͨ��CO����Ӧ��ʼ����
��b���ʾͨ��COһ��ʱ�����ȣ���Ӧ��ʼ����
��c���ʾ��ɫ��ĩ�ѳ�ֲμӷ�Ӧ
��d���ʾ��Ӧ�������������ͨ��CO
�ݿɸ���ͨ��CO�������������ɫ��ĩ������
(3)ͼ1װ���еIJ���֮���� ��
(4)��ʵ�����Ƶ����빤ҵ���Ƴ��������������� ��
(5)ij��ѧС���ͬѧ����������ʵ���һ�ݹ�����Ʒ������̽����ͨ��ʵ����ȷ������Ʒ�������������ۻ�϶��ɡ�����ȡ��3.6g������Ʒ����ͼ1��ʾ��װ������ʵ�飬�ⶨ�IJ���������ͼ3��ʾ����ԭ��Ʒ����Ԫ������Ԫ�ص��������� ��
��1��Fe2O3+3CO2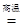 Fe+3CO2����2���٢ݣ���3��û�ж�β�����д�������4����ʵ�����Ƶ����Ǵ������ҵ���Ƶ������ǻ�������һ������̼����5��5��1
Fe+3CO2����2���٢ݣ���3��û�ж�β�����д�������4����ʵ�����Ƶ����Ǵ������ҵ���Ƶ������ǻ�������һ������̼����5��5��1
����������1����ɫ��ĩ������������������һ����̼�ڸ����������������Ͷ�����̼������ʽ�ǣ�Fe2O3+3CO2=����=Fe+3CO2��
��2��ʵ�鿪ʼ��ͨ��һ����̼���ž��Թ��ڿ�������ֹ���ȷ�����ը����Ӧ���Ҫ����ͨһ����̼����ֹ���ɵ�����������������һ����̼��δ������Ӧ�����Բ��ܸ���ͨ��һ����̼������ ���м��㣬a-b��ɫ��ĩ������δ�䣬˵����Ӧ��δ��ʼ��
��3��һ����̼�ж���δ������Ӧ��һ����̼Ҫ���д�����ͼ��ȱ��β������װ�ã�
��4����ҵ���Ƶ�������������һ������̼���˴����Ƶ��Ǵ���������һ���Ǵ����һ���ǻ�����
��5��һ����̼����������Ӧ�������Ͷ�����̼�����Բ����ܹ�����ٵ�����������������Ԫ�ص�������
�⣺��������������Ϊx��
Fe2O3+3CO2=����=Fe+3CO2 ������ٵ�����
160 �� 112 160-112=48
x 3.6g-3g ��
x=2g
������Ʒ�����۵�������3.6g-2g =1.6g
��Ʒ��FeԪ����������1.6g+2g��112/160��100%=3g
OԪ����������2g��48/160��100%=0.6g
������Ʒ����Ԫ������Ԫ�ص��������ǣ�3g��0.6g=5��1
�𣺸���Ʒ����Ԫ������Ԫ�ص���������5��1��


 ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(5��)������ұ��������������һ����Ҫ��־��ͼ1��ʵ����ģ��������װ��ͼ��


ͼһ ͼ�� ͼ��
(1)д��ͼ1��A�������Ļ�ѧ��Ӧ����ʽ�� ��
(2)ʵ�������ͨ��CO�������ɫ��ĩ�����Ĺ�ϵ��ͼ2��ʾ������ͼʾ������˵������ȷ���� ��������ţ�
��a���ʾ��ʼͨ��CO����Ӧ��ʼ����
��b���ʾͨ��COһ��ʱ�����ȣ���Ӧ��ʼ����
��c���ʾ��ɫ��ĩ�ѳ�ֲμӷ�Ӧ
��d���ʾ��Ӧ�������������ͨ��CO
�ݿɸ���ͨ��CO�������������ɫ��ĩ������
(3)ͼ1װ���еIJ���֮���� ��
(4)��ʵ�����Ƶ����빤ҵ���Ƴ��������������� ��
(5)ij��ѧС���ͬѧ����������ʵ���һ�ݹ�����Ʒ������̽����ͨ��ʵ����ȷ������Ʒ�������������ۻ�϶��ɡ�����ȡ��3.6g������Ʒ����ͼ1��ʾ��װ������ʵ�飬�ⶨ�IJ���������ͼ3��ʾ����ԭ��Ʒ����Ԫ������Ԫ�ص��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(5��)������ұ��������������һ����Ҫ��־��ͼ1��ʵ����ģ��������װ��ͼ��



ͼһ ͼ�� ͼ��
(1)д��ͼ1��A�������Ļ�ѧ��Ӧ����ʽ�� ��
(2)ʵ�������ͨ��CO�������ɫ��ĩ�����Ĺ�ϵ��ͼ2��ʾ������ͼʾ������˵������ȷ���� ��������ţ�
��a���ʾ��ʼͨ��CO����Ӧ��ʼ����
��b���ʾͨ��COһ��ʱ�����ȣ���Ӧ��ʼ����
��c���ʾ��ɫ��ĩ�ѳ�ֲμӷ�Ӧ
��d���ʾ��Ӧ�������������ͨ��CO
�ݿɸ���ͨ��CO�������������ɫ��ĩ������
(3)ͼ1װ���еIJ���֮���� ��
(4)��ʵ�����Ƶ����빤ҵ���Ƴ��������������� ��
(5)ij��ѧС���ͬѧ����������ʵ���һ�ݹ�����Ʒ������̽����ͨ��ʵ����ȷ������Ʒ�������������ۻ�϶��ɡ�����ȡ��3.6g������Ʒ����ͼ1��ʾ��װ������ʵ�飬�ⶨ�IJ���������ͼ3��ʾ����ԭ��Ʒ����Ԫ������Ԫ�ص��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������������һ�о��꼶��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�̽����
(5��)������ұ��������������һ����Ҫ��־��ͼ1��ʵ����ģ��������װ��ͼ��

ͼһ ͼ�� ͼ��
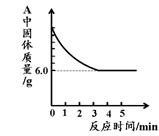
(1)д��ͼ1��A�������Ļ�ѧ��Ӧ����ʽ�� ��
(2)ʵ�������ͨ��CO�������ɫ��ĩ�����Ĺ�ϵ��ͼ2��ʾ������ͼʾ������˵������ȷ���� ��������ţ�
��a���ʾ��ʼͨ��CO����Ӧ��ʼ����
��b���ʾͨ��COһ��ʱ�����ȣ���Ӧ��ʼ����
��c���ʾ��ɫ��ĩ�ѳ�ֲμӷ�Ӧ
��d���ʾ��Ӧ�������������ͨ��CO
�ݿɸ���ͨ��CO�������������ɫ��ĩ������
(3)ͼ1װ���еIJ���֮���� ��
(4)��ʵ�����Ƶ����빤ҵ���Ƴ��������������� ��
(5)ij��ѧС���ͬѧ����������ʵ���һ�ݹ�����Ʒ������̽����ͨ��ʵ����ȷ������Ʒ�������������ۻ�϶��ɡ�����ȡ��3.6g������Ʒ����ͼ1��ʾ��װ������ʵ�飬�ⶨ�IJ���������ͼ3��ʾ����ԭ��Ʒ����Ԫ������Ԫ�ص��������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com