
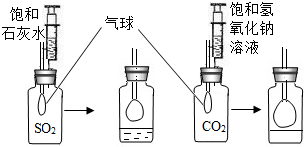
ijУѧ������������������Һ��������ƿ�������ڸ���Һ�Ƿ���ʣ�������������¼��裺
����һ������Һû�б���
�����������Һ���ֱ���
������������Һȫ������
�����������������Һ���ʵ�������________________________________________________��
��.�����о�
Ϊ����֤���ϼ��裬����ͬѧ�������������ʵ�鷽��������ʾ���Ȼ�����Һ�����ԣ�
| ʵ�鲽�� | ʵ������ | ���� | |
| ����1 | ȡ��������������Һ�����Թ��У�����2����ɫ��̪��Һ | ��ɫ��̪��Һ��� | ����һ���� |
| ����2 | ȡ��������������Һ�����Թ��У��������BaCl2��Һ���ٵ�����ɫ��̪��Һ | �а�ɫ������������̪��� | ����_____���� |
| �а�ɫ������������̪����� | ����_____���� |
���������ۡ�
����ͬѧ��Ϊ���ǵ�ʵ�鷽�������ܣ�����һ����֤ʵ��Һû�б��ʡ����о�������һ�Ľ����£�ȡ��������Һ���Թ��У�����������____________���������ݲ����������һ������
���ͬѧ�ǹ�ͬŬ�����ó����ۣ�������������������Ǽ����о���ȥ��
��.����̽��
��������⡿��ƿ��Һ��̼���Ƶ������Ƕ����أ�
�����ʵ�顿ͬѧ�����ۺ���ΪֻҪ�ռ�����ʵ���е�������ݣ�ͨ������Ϳ��Եó���Ʒ��̼���Ƶ�����������Ϊ�����������_____________��
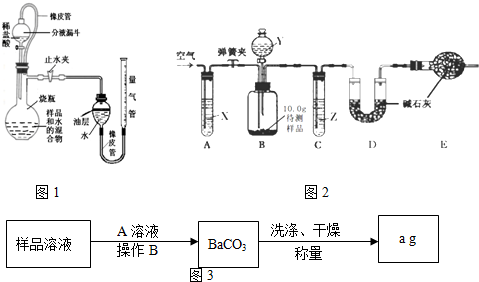
��1���ⶨ������̼�����������ͬѧ�������ͼ��ʾ��ʵ��װ�ã�����̨�ȹ̶�װ������ȥ����ȡ������Ʒ������ʵ�顣
 ��ʵ�鲽�衿
��ʵ�鲽�衿
����ͼ��ʾ�����Ӻ�װ�ã����װ���� �������ã�
�������ã�
�ڳ�һ��������Ʒ�����ձ��У���������ˮ��ϣ���ȫ�ܽ��ȫ��������ͼ����ƿ�У����Һ©���м�������ϡ������ã�
�۽�������Һ����ڵ���0���̶ȣ���ʹ��������Һ�����Ͳ���ƽ����ֹˮ�кͷ�Һ©��������
�ܷ�Ӧ�������ٴε�����������Һ�����Ͳ�Һ����ƽ���������������
�ݲ��CO2���ܶȣ�������Ʒ��̼���Ƶ�����������
���������ۡ�
ͼ���Ͳ������Ϊ ���Ͳ��Ϸ����п�������ʹ������� ���ƫ����ƫС������Ӱ�족�����кͷ�Ӧ���Էų��������μ�ϡ�������װ��������кͷ�Ӧ�Ļ�ѧ����ʽΪ �������Ӧ�ķ�������ʹ�ⶨ��� ���ƫ����ƫС������Ӱ�족����
��2���ⶨ������̼������������ͬѧ����˿���������װ�òⶨ������̼������������̨�����еȹ̶���װ������ȥ����ʯ���ǹ����������ƺ���ʯ�ҵĻ�����
ȡ10.0g������Ʒ������ʵ�飮ʵ�鲽�����£�
a�����Ӻ�װ�ã�����������ԣ�
b�����ɼУ�����ͨ��һ��ʱ�������
c������װ��D������Ϊ83.4g��
d���رյ��ɼУ������μ�Y��Һ�������ٲ�������Ϊֹ��
e�����ɼУ��ٴλ���ͨ��һ��ʱ�������
 f���ٴγ���װ��D������Ϊ84.5g��
f���ٴγ���װ��D������Ϊ84.5g��
���������ۡ�
���Լ�X��Y��Z����������ѡ��___________��ѡ����ĸ��
A������������Һ Ũ���� Ũ���� B������ʯ��ˮ ϡ���� ϡ����
C������������Һ ϡ���� Ũ���� D��Ũ���� ϡ���� ����������Һ
�ڲ���e��ͨ�������������______________________________________��
����û��װ��C����ᵼ�²ⶨ���________��ѡ�ƫ����ƫС������Ӱ�족����ͬ������û��װ��E����ᵼ�²ⶨ���_________��
���������ۡ�����ʵ���в�õ��й����ݣ����㲿�ֱ��ʵ��������ƹ�����̼���Ƶ�����������
��3������ͬѧ��������������·����ⶨ̼���Ƶ�����

�ٸ�ʵ��Ҫ������A��Һ������������A��Һ�ѹ����ķ�����________________________
�ڲ���B��������____________________���ò����õ��IJ���������______________________��
��������������еĶ�����̼��Ӧ
��.�����о� �� �� ϡ����
��.����̽�� ���ɶ�����̼����������� ��1�����������ۡ�����CO2��ˮ����ֹCO2����ˮ����ˮ��Ӧʽ ����Ӱ�� NaOH+HCl===NaCl+H2O ƫ��
��2��C ʹװ���е�CO2ȫ������ʯ�������� ƫ�� ƫ��
�⣺��̼��������Ϊx
Na2CO3+2HCl===2NaCl+H2O+ CO2��
106 44
x 84.5g-83.4g=1.1g
106/44=x/1.1g x=2.65g 2.65g/10g��100%=26.5% ����
��3��ȡ��Һ�����м���Na2CO3��Һ�����ɰ�ɫ����������������Ҳ�ɣ�
���� ������ �ձ� ©��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijУѧ����ʵ�����������������ȼ�յ�����ʵ��ʱ����ʼ������������ɫ�����ˣ���������������һ��ʱ���ͬѧ�Dz��ò��˳����ң���Ϊ���ڳ�����Ũ�صĴ̼�����ζ��
ijУѧ����ʵ�����������������ȼ�յ�����ʵ��ʱ����ʼ������������ɫ�����ˣ���������������һ��ʱ���ͬѧ�Dz��ò��˳����ң���Ϊ���ڳ�����Ũ�صĴ̼�����ζ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ�鲽�� | ʵ������ | ���� | |
| ����1 | ȡ��������������Һ�����Թ��У�����2����ɫ��̪��Һ | ��ɫ��̪��Һ��� | ����һ���� |
| ����2 | ȡ��������������Һ�����Թ��У��������BaCl2��Һ���ٵ�����ɫ��̪��Һ | �а�ɫ������������̪��� | ���� |
| �а�ɫ������������̪����� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�鲽�� | ʵ������ | ���� | |
| ����1 | ȡ��������������Һ�����Թ��У�����2����ɫ��̪��Һ | ��ɫ��̪��Һ��� | ����һ���� |
| ����2 | ȡ��������������Һ�����Թ��У��������BaCl2��Һ���ٵ�����ɫ��̪��Һ | �а�ɫ������������̪��� | ����______���� |
| �а�ɫ������������̪����� | ����______���� |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com