
�ҹ��Ƽҵ������������°�̽�������ˡ������Ƽ���������������漰����Ҫ��ѧ��Ӧ���£�
��NH2��CO2��X��NH4HCO3[
��NH4HCO3��NaCl��NH4Cl��NaHCO3��
��2NaHCO3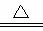 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��ش��������⣺
��1����Ӧ����X�Ļ�ѧʽΪ��
��2����ȥ����Na2CO3��ĩ��������NaHCO3�ķ����� ��
��3����ҵ�����к����Ȼ��ƣ�ȡ55g��ҵ��������м���269.5gϡ���ᣬǡ����ȫ��Ӧ������22g������̼����
�ٹ�ҵ������̼���Ƶ�������������������������0.1%��
�ڷ�Ӧ����Һ�����ʵ�����������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊʵ������ʵ��Ŀ�ģ���ѡ���Լ�����ȷ���ǣ���
ѡ�� ʵ��Ŀ�� �����Լ���
A�� ����NH4NO3��Һ��K2SO4��Һ ��Ba��NO3��2����Һ
B�� ��ȥ��ʯ���к��е�����ʯ��ʯ ��ˮ��ϡ����
C�� ��ȥCO2�е�CO���� �������е�ȼ
D�� ��ȥ����ͭ��ĩ�е�ͭ�ۼ�������ϡ���ᣬ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й������漰��ѧ�仯���ǣ�������
| �� | A�� | �þ���ϩ�����Ƶ�ʳƷ�� | B�� | ��ʳ���Ƶô��� |
| �� | C�� | ��Һ̬���������Ƶ����� | D�� | ��ʯ�ͷ����Ƶ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ȡ10g̼�����������������Ϊ10%��ϡ���ᷴӦ��ǡ����ȫ��Ӧʱ��õ���Һ������Ϊa g����ȡ10g̼��Ƹ�����ȫ�ֽ��ȡ���ɵ�CaO������ͬŨ�ȵ�ϡ���ᷴӦ��ǡ����ȫ��Ӧʱ�õ���Һ������Ϊb g����a��b�Ĵ�С��ϵΪ����
��A�� a=b B�� a��b C�� a��b D�� ����ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С����ʵ���ҷ���һƿδ֪Ũ�ȵ�����������Һ��Ϊ�����Ժ�ʹ�ã�������Ũ�Ƚ����˲ⶨ��ȡ20.0g������������Һ���ձ��У���εμ�������������Ϊ7.3%��ϡ���ᣬ����ʱ�Է�Ӧ�����Һ��pH�ƣ�һ�ֲⶨ��ҺpH���������ⶨ��Һ��pH���������������
| ����ϡ���� ������/g | 9.6 | 9.8 | 9.9 | 10.0 | 10.1 |
| ��Һ��pH | 12.4 | 12.1 | 11.8 | 7.0 | 2.2 |
�Իش�
��1�����μ�ϡ���������Ϊ9.8gʱ����Һ�е�����������������
��2��������������������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڻ�ѧ��Ӧ2X��Y===2Z������������ȷ����(����)��
A��Zһ���ǻ�������ҿ�����������
B���ڷ�Ӧ��X��Y��Z�������ʵ�������Ŀ��Ϊ2��1��2
C����X��Y����Է��������ֱ�ΪM��N����Z����Է�������Ϊ(M��N)
D����Ӧ���ڻ��Ϸ�Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͨ�����ǵĻ��������(N2H4)��ȼ�ϣ�������������(N2O4)��ȼ�������ﲻ��Դ��������Ⱦ��
(1)��Ӧ�Ļ�ѧ����ʽΪ2N2H4��N2O4===3________��4H2O�����ں�������д��ѧʽ����ɸû�ѧ����ʽ��
(2)�����9.6 kg N2H4��ȫȼ����Ҫ��ȼ��N2O4��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ʋ�ϡ��һ������������Na2SO4��Һ��
��1������50g��������Ϊ6%��Na2SO4��Һ��
�ټ��㣺��ҪNa2SO4 3.0g��ˮ 47.0g
�ڳ�������������ƽ����3.0g��Na2SO4����ƽ����ֱ�����ƽ�������̷���������ͬ��ֽƬ����������Ȼ����������������ƽǡ��ƽ�⣮
����ȡ������Ͳ��ȡ47.0mLˮ��������ͼ�л���47.0mLˮ��Һ��λ�ã�
���ܽ⣮
��2��ϡ����Һ���������������ƹ�������Һ��ϡ�����ܶȿɽ��ƿ���1g/mL��
��ȡ1mL 6%��Na2SO4��Һ��ˮϡ����100mL���õ���Һa��
������3.0gNa2SO4��������ҺaŨ����ͬ����Һ�����������mL��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������Ǵ����˷��㣬ͼʾ����ͨ���Ӵ�����ʵ����Ƭ�����������������ȼ�������ܴ��������(��)

A���٢ۢ� B���٢ۢ� C���٢ڢ� D���٢ڢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com