
��ѧ��ȤС���ij��ҵ��ˮ������ΪHCl��NaCl���е�HCl�������вⶨ���ס�����λͬѧ���ṩ��ͬ�IJⶨ������
��1����ͬѧ������кͷ�
ȡ50g��ˮ���ձ��У���ε���������������Ϊ10%��NaOH��Һ����Ӧ��������Һ��pH�仯��ͼ��ʾ�����ˮ��HCl������������д����ϸ�ļ�����̣���

��2����ͬѧ��������
����AgNO3��Һ����NaOH��Һ���������ɳ�����������ȷ����ˮ��HCl����������������Ϊ�����_________���ƫ��ƫС������Ӱ�족��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������2018����꼶�п���Ϣ����ѧ�Ծ� ���ͣ�ѡ�������
��ͼ�Ǽס��ҡ����������ʵ��ܽ�����ߣ�����˵������ȷ����
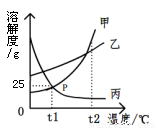
A. P���ʾ�ס����������ʵı�����Һ�������
B. t1��ʱ�������ʵı�����Һ��������t2��ʱ���DZ�����Һ
C. t1��ʱ�������ʵı�����Һ�����ʺ��ܼ���������Ϊ1��4
D. ���������ʵ���Һ��t2�潵��t1�棬��������������С��һ���DZ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2018����꼶��ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ���Ϣ������
���������У����ڴ�������� ��________��
A�������ʯ��ˮ B�����ʵĿ��� C��������̼ D��_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ����������ѧ��2018����꼶��ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ѡ�������
ʵ�����ø��������ȡ��������Ҫ�����У���װҩƷ���ڼ��װ�������ԣ��۹̶�װ�ã��ܼ��ȣ����ռ����壻���ƾ��ƣ��ߴ�ˮ���г������ܡ���ȷ�IJ���˳��Ϊ �� ��
A. �ڢ٢ۢܢݢޢ� B. �٢ڢۢܢݢޢ�
C. �٢ڢۢܢݢߢ� D. �ڢ٢ۢܢݢߢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ����������ѧ��2018����꼶��ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ѡ�������
�ҹ������ġ�����������������������PM2.5�ļ��ָ�ꡣPM2.5��ֱָ����2.5�����µ�ϸ������������彡���ͻ��������кܴ��Ӱ�졣���д�ʩ��PM2.5���������������õ��� �� ��
A. ��ǿ�������ص��ﳾ���� B. ���е�·��ʱ��ˮ
C. ����ֲ������ D. ������չ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�ij���ݷ��2017����꼶���Ĵ�ģ�⿼�Ի�ѧ�Ծ� ���ͣ���Ϣ������
��Ƕ���ʶ���ʵı仯�����������Ǹ��õ����⻯ѧ֪ʶ��
��1�����۽Ƕ�:��ͼ1Ϊij��ѧ��Ӧ����ʾ��ͼ����ͼ�ش��������⣩

�����Ͽ����û�ѧ�仯�з��������ı������___________��ѡ�ԭ�ӡ����ӡ�����
�ڲμӷ�Ӧ��A2��B2�������ʵķ��Ӹ�����Ϊ_______________��
��2���������仯�Ƕ�:������ͼ2װ�÷ֱ��������ʵ�飬��ʶ���ʱ仯�е������仯��
�����ձ��м���һ������ʯ�һ��������ȼ�գ���ȼ��������������ʱ��ʯ�ҵ�������_______________��
�ڽ�һ����������ij���������ձ��е���ˮ���������������ȼ�յ���______��ѡ����ĸ��ţ���
A.�Ȼ��� B.�����
C.Ũ���� D.��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�ij���ݷ��2017����꼶���Ĵ�ģ�⿼�Ի�ѧ�Ծ� ���ͣ�ѡ�������
ijЩ�����⻯����ˮ��Ӧ�����ɼ����������CaH2+2H2O�TCa��OH��2+2H2����NaH��CaH2�Ļ�ѧ�������ƣ���NaH����������ϡ�����У����ɵ�������Ϊ�� ��
A. NaOH��H2
B. NaOH��NaCl
C. NaOH��H2��NaCl
D. NaCl��H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������У2018����꼶��ѧ�ڵ�һѧ�¿��Ի�ѧ�Ծ� ���ͣ���Ϣ������
д�����з�Ӧ�����ֱ���ʽ����ű���ʽ��
�ź����ڿ�����ȼ��__________________________________________________________��
��̼��������ȼ��__________________________________________________________��
������������ȼ��___________________________________________________________��
������������ȼ��____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡТ���С�ë����ѧ2018����꼶��ѧ�ڵ�һ��������ѧ�Ծ� ���ͣ������
ijʵ�������˺����ĸ������������������±���ʾ��
���� | �������� | �������� |
X | 78% | 75% |
Y | 21% | 15% |
������̼ | 0.03% | 3.68% |
ˮ���� | 0.06% | 5.44% |
���� | 0.91% | 0.88% |
��1�������жϣ�X��___________��Y��___________��
��2������ش�����������³´�л�����ĵ�������_____________��
��3����֤���������������к���ˮ���������ʵ�鷽����___________________
��4���������X�����ں���������û���뻯ѧ��Ӧ�����ں������������������ȴ�����ˣ�ԭ����____________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com