
��ͼ��A��B�ֱ���ij���Ľṹʾ��ͼ,��ش��������⣺

(1)��A��ijԭ�ӵĽṹʾ��ͼ,��x=____ ��
(2)ͼ��BԪ������_____����������ǽ�������Ԫ�أ�
(3)ͼA����x=13,��A��B����ʾ��Ԫ���γɻ�����Ļ�ѧʽΪ_______��
(4)ͼA����x=12,���Ԫ��λ��Ԫ�����ڱ���_____���ڡ�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ס��ҡ��������ֱ���ʯ����Һ��ϡ���ᡢ̼������Һ������ʯ��ˮ�е�һ�֣������ĸ�Բ��ʾ������Һ����Բ�ཻ����Ϊ����Ҳ��Ϻ���ֵ���Ҫʵ��������ͼ��ʾ����ش�
�ס��ҡ��������ֱ���ʯ����Һ��ϡ���ᡢ̼������Һ������ʯ��ˮ�е�һ�֣������ĸ�Բ��ʾ������Һ����Բ�ཻ����Ϊ����Ҳ��Ϻ���ֵ���Ҫʵ��������ͼ��ʾ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��ͷ�����������꼶5��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
��ѧ��Tim Richard�����ӽṹ��ʽ��С����ij�л���(��ͼ��ʾ)��ȡ��Ϊ��С��ϩ��(��ѧʽΪC26H26)������㣺
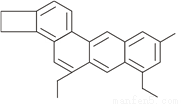
(1)��С��ϩ������Է���������__________��
(2)��С��ϩ����̼Ԫ�غ���Ԫ�ص�������_____________(�����������)��
(3)16.9g��С��ϩ���к�̼Ԫ��___________�ˡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ��ͷ�����������꼶5��ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
Sb2O5 ��һ����Ҫ����ȼ������ҵ��ȡ�����ʵĻ�ѧ����ʽΪ��Sb2O3 + 2X�TSb2O5 + 2H2O���� X �Ļ�ѧʽΪ�� ��
A��H2 B��O2 C��H2O2 D��H3SbO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ���������ؾ��꼶��ѧ�ڵڶ���ѧҵˮƽģ����Ի�ѧ�Ծ��������棩 ���ͣ���Ϣ������
ȡCaCl2��CaCO3�Ļ����12.5g���ձ��У������еμ�һ����������������ϡ���ᣬ�μ�ϡ�����������������������Ĺ�ϵ��ͼ��ʾ����
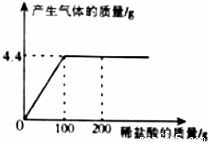
��1���������CaCO3������Ϊ____g��
��2��ǡ����ȫ��Ӧʱ�����ò�������Һ�����ʵ�������������д��������̣���������ȷ��0.1%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ���������ؾ��꼶��ѧ�ڵڶ���ѧҵˮƽģ����Ի�ѧ�Ծ��������棩 ���ͣ�ѡ�������
С��ͬѧ����������д�Ļ�ѧ���ţ������������ӣ�2N2������������ԭ�ӹ��ɵij������ӣ�3O�������ӽṹʾ��ͼ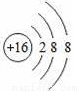 ��ʾ�����ӣ�S�������������ӣ�2Fe3+���ݵ��硢��������õĽ�����Ag����+2�۵�þԪ�أ�Mg2+��������л������C2H5OH�����У���ȷ���ǣ� ��
��ʾ�����ӣ�S�������������ӣ�2Fe3+���ݵ��硢��������õĽ�����Ag����+2�۵�þԪ�أ�Mg2+��������л������C2H5OH�����У���ȷ���ǣ� ��
A. �ڢܢ� B. �٢ܢ� C. �٢ۢ� D. �٢ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ���������пƬ����Ƭ�ֱ���������ĵ�����������ϡ���� | |
| B�� | ��H2SO4��CuSO4�Ļ����Һ�еμ�NaOH��Һ | |
| C�� | ��һ������ϡ�����м���п�� | |
| D�� | ���ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����������Һ��Ͷ��һС������ƣ���Ӧ���ң�������ͼ����
����������Һ��Ͷ��һС������ƣ���Ӧ���ң�������ͼ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
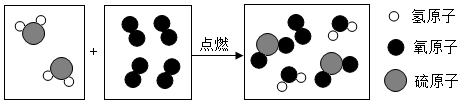
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com