
| ||
| С���Ľ��ⷽ�� | С�ݵĽ��ⷽ�� | ||||||
| �⣺����������Ϊx 2Al2O3
204 108 10t x 204��108=10t��x x=5.3t ������������5.3t |
�⣺����������Ԫ�ص���������Ϊ��
����������10t��53%=5.3t ����������5.3t |
| ||
| 2��158 |
| 32 |
| 15.5g |
| X |


 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| С��ͬѧ�Ľⷨ | С��ͬѧ�Ľⷨ | |||||||||||||||||||||||||
| �⣺����������Ϊx 2Al2O3
204 108 10t x
����������5.3t���� |
�⣺����������Ԫ�ص�����������
����������lOt��53%=5.3t ����������5.3t���� |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
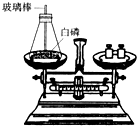 ��1����ͼ��ʾΪ�ü��Ⱥ�IJ�������ȼ���ף����ⶨ����ȼ��ǰ�������ı仯�����������֤�����غ㶨�ɵ�ʵ�飮
��1����ͼ��ʾΪ�ü��Ⱥ�IJ�������ȼ���ף����ⶨ����ȼ��ǰ�������ı仯�����������֤�����غ㶨�ɵ�ʵ�飮
| ||
| Сٻͬѧ�Ľⷨ | С��ͬѧ�Ľⷨ | ||||||||||||
| �⣺�����ɵ�������ΪX 2Al2O3
204108 10t X
X=5.3t ����������5.3t�� |
�⣺����������Ԫ�ص���������Ϊ
��������Ϊ10t��53%=5.3t ����������5.3t�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com