
| ���� �����500mL ��ѧʽ��HCl ��Է���������36.5 �ܶȣ�1.1g/cm3 ����������30% |
 ��88.3%
��88.3% =20%
=20%

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� �����500mL ��ѧʽ��HCl ��Է���������36.5 �ܶȣ�1.1g/cm3 ����������30% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ�ϵ����ݻش����⣺
����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ�ϵ����ݻش����⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
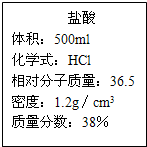 ����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ����������⣺
����һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ����������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� �����500mL ��ѧʽ��HCl ��Է���������36.5 �ܶȣ�1.2g/cm3 ����������37%��1���ø�Ũ����100ml��������������Ϊ20%������ ��2�����ø����Ƶ�ϡ�������ⶨij̼������Ʒ�Ĵ��ȣ���Ʒ������Ԫ�أ���ȡ50g����Ʒ�������е������õ�ϡ���������ٲ�������Ϊֹ������ȥ����146g�� �ٷ�����Ӧ�Ļ�ѧ����ʽ ��������֪�������μӷ�Ӧ�Ĺ�������������x���ı���ʽ �۸���Ʒ�ijɷ��� �ܸ���Ʒ�Ĵ����� ������Ӧ����Һ�м���46.8gˮ��ʱ������Һ�����ʵ���������Ϊ �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ�  ��1����������炙�ѧʽΪNH4NO3������NԪ�صĻ��ϼ�Ϊ ��1����������炙�ѧʽΪNH4NO3������NԪ�صĻ��ϼ�Ϊ-3��+5 -3��+5 ���䵪Ԫ�ء���Ԫ�ء���Ԫ��������Ϊ7��1��12 7��1��12 ����2������һƿ��ǩ��ͼ��ʾ��Ũ���ᣬ����ݱ�ǩ�ϵ����ݻش����⣺ ��Ҫ����165g20%�����ᣬ���ø�Ũ���� 100 100 mL����Ϊ�Ӷ����Ƕ���ʶ�кͷ�Ӧ��С��ͬѧ������Ʋ�����������ʵ�飺 ������С�ձ��е���8%������������Һl0g������2�η�̪�Լ�����Һ�ʺ�ɫ�� ������ȡ10%��ϡ������εμӵ�����������Һ�У��ߵα�����������룬��Һ��ɫ��dz�� ��������Һ��ɫ��ʧ˲�䣬ֹͣʵ�飮 ��ֹͣʵ��ʱ������Һǡ�÷�Ӧ�������С��ʵ���ش� A��ʵ���з�̪�Լ��������� �ж��кͷ�Ӧ���еij̶� �ж��кͷ�Ӧ���еij̶� ��B���������ʱ����ȥϡ����������Ƕ��٣�����ȷ��0.1�� �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ѧУ��ѡ - ��ϰ���б� - �����б� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |