
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�������ˮ�ĵ��룩��K����NH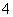 ��Cl����Mg2����Ba2����CO
��Cl����Mg2����Ba2����CO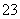 ��SO
��SO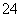 ����ȡ����100mL��Һ��������ʵ�飺��1����һ�ݼ���AgNO3��Һ�г�����������2���ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�����״��������896ml������ȫ���ݳ�������3�������ݼ�����BaCl2��Һ
����ȡ����100mL��Һ��������ʵ�飺��1����һ�ݼ���AgNO3��Һ�г�����������2���ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�����״��������896ml������ȫ���ݳ�������3�������ݼ�����BaCl2��Һ
�ø������6.63g������������ϴ�ӡ������������4.66g����������ʵ�飬
�����Ʋ���ȷ����
A�� K��һ������ B�� 100 mL��Һ�к�0.02 mol CO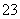
C�� Cl��һ������ D�� Ba2��һ�������ڣ�Mg2�����ܴ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪2H2��g����O2��g��= 2H2O��l�� ��H=��569��6 kJ��mol��1�� 2H2O��g��= 2H2��g����O2��g�� ��H=��482��1 kJ��mol��1������1 gҺ̬H2O������ʱ���յ�������
A��2��43 kJ B��4��86 kJ C��43��8 kJ D��87��5 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���ӽṹʾ��ͼΪ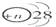 ����������Ϊ���ӣ�������ԭ����������Ϊ(�� ��)
����������Ϊ���ӣ�������ԭ����������Ϊ(�� ��)
��11 ��14�� �� 9�� �� 8
A���٢ڢ� B���٢ۢ� C���ۢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ǷŴ��˵ط�����Դ��������Ӧ����������á������з��������ϴ�����ֽ��������Ʒ������
A������ B����� C������ D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�������£����淴Ӧ2X(g)+3Y(g) 4Z(g),��X��Y��Z��ʼŨ�ȷֱ�Ϊc1��c2��c3(����Ϊ0)��ƽ��ʱX��Y��Z��Ũ�ȷֱ�Ϊ0.2mol��L��1��0.3 mol��L��1��0.16 mol��L��1���������жϲ���������
4Z(g),��X��Y��Z��ʼŨ�ȷֱ�Ϊc1��c2��c3(����Ϊ0)��ƽ��ʱX��Y��Z��Ũ�ȷֱ�Ϊ0.2mol��L��1��0.3 mol��L��1��0.16 mol��L��1���������жϲ���������
A��c1��c2=2��3 B��ƽ��ʱ��Y��Z����������֮��Ϊ3��4
C�� X��Y��ת������� D�� c3��ȡֵ��ΧΪ0��c3��0.28 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڵ��۵⻯����Һ�м�������NaClO��Һ������Һ��������������Һ�м���������Na2SO3��Һ����ɫ����ʧ�������жϴ������
A�������ԣ�ClO����I2
B����ɫ����ʧ˵��Na2SO3����Ư����
C��Ư����Һ��ʹ���۵⻯����ֽ����
D����������ˮ�м�����������������Һ����ˮ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��þ3����5����þ���Ͻ����ѳ�Ϊ�ִ����졢������������е�������ҵ����Ҫԭ���ϡ�����һ����֪����Ϊ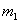 g��þ���Ͻ����ⶨ����þ��������������λͬѧ������������ֲ�ͬ��ʵ�鷽����
g��þ���Ͻ����ⶨ����þ��������������λͬѧ������������ֲ�ͬ��ʵ�鷽����
ʵ�����1��þ���Ͻ�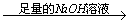 ��ַ�Ӧ��ⶨʣ���������
��ַ�Ӧ��ⶨʣ���������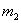 g
g
ʵ�����2��þ���Ͻ� ���ɵ������ڱ�״���µ����ΪVL��
���ɵ������ڱ�״���µ����ΪVL��
��ش��������⣺
��1��ʵ�����1��
�����ܽ⡢������ʹ�õIJ����������ձ���� ��
��þ������������ ��������˵õ�����û��������ˮϴ�����κ��ɣ��ٲⶨʣ�������������þ������������ ���ƫ����ƫС������Ӱ�족����
��2��ʵ�����2���ⶨ���ɵ�����װ������ͼ,���еIJ����У�
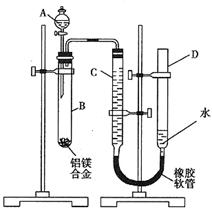 a����¼C��Һ��λ�ã�
a����¼C��Һ��λ�ã�
b����A��B�еμ������Լ���
c����B�в���������������ָ������º�¼
C��Һ��λ�ã�
d����������ԣ�
e. ��ҩƷ��ˮװ��������У����Ӻã�
������������˳���ǣ�________________������ţ���
�ڼ�¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧע�⣺
_____________ ��

 ��3����������ʵ�����1��ʵ�����2��ģʽ�����һ��ʵ�鷽���ⶨ����þ������������
��3����������ʵ�����1��ʵ�����2��ģʽ�����һ��ʵ�鷽���ⶨ����þ������������

 ��þ�Ͻ� ��Һ ���ˣ��ⶨ������
��þ�Ͻ� ��Һ ���ˣ��ⶨ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪���� S(��б��s)��O2(g) === SO2(g) ��H1����297.16 kJ��mol-1
�� S(������s)��O2(g) === SO2(g) ��H2����296.83 kJ��mol-1
�� S(���s) === S(������s) ��H3
����˵����ȷ���ǣ� ��
A����H3����0.33 kJ��mol-1��������
B����б��ת��Ϊ������ķ�Ӧ�����ȷ�Ӧ
C��S����б��s��=== S��������s�� ��H3��0��������ȵ�б���ȶ�
D��S����б��s��=== S��������s�� ��H3��0����б����������ȶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[��ѧ����ѡ��2����ѧ�뼼��](15��)
����ˮ������Ũ��ˮ��Դ������������ۺ����ú�ˮ����Ҫ;��֮һ��һ�����Ƚ���ˮ������õ�ˮ���ٴ�ʣ���Ũ��ˮ��ͨ��һϵ�й���������ȡ������Ʒ��
�ش��������⣺
(1)���иĽ����Ż���ˮ�ۺ����ù��յ�������������е���________(�����)��
���û�������ȡ��ˮ
����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ��
�ܸĽ��ء��塢þ�ȵ���ȡ����
(2)���á���������������Ũ��ˮ����Br2�����ô������ա������������Ҫ��Ӧ��Br2��Na2CO3��H2O��NaBr��NaBrO3��NaHCO3������1 mol Br2ʱ��ת�Ƶĵ�����Ϊ________mol��
(3)��ˮ��þ��һ�ι�����������ͼ��

Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na�� | Mg2�� | Cl�� | SO |
| Ũ��/(g��L��1) | 63.7 | 28.8 | 144.6 | 46.4 |
�ù��չ����У��������Ҫ��Ӧ�����ӷ���ʽΪ______________________________����Ʒ2�Ļ�ѧʽΪ__________��1 LŨ��ˮ���ɵõ���Ʒ2������Ϊ________g��
(4)����ʯī���������������������ڵ��Ȼ�þ��������Ӧ�Ļ�ѧ����ʽΪ________________________�����ʱ����������ˮ���ڻ���ɲ�Ʒþ�����ģ�д���йط�Ӧ�Ļ�ѧ����ʽ��____________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com