
ijͬѧ�������ϵ�֪���ٲ��ᾧ��(H2C2O4��2H2O)��175��ʱ���ȷֽ⣬ͬʱ�ۻ�������ʱ�ӷ����ڲ��ᾧ��������ˮ���������������ˮ��
Ϊ��֤����ֽ�IJ����ͬѧѡ�������������Լ��еIJ�����ɴ�ʵ�顣
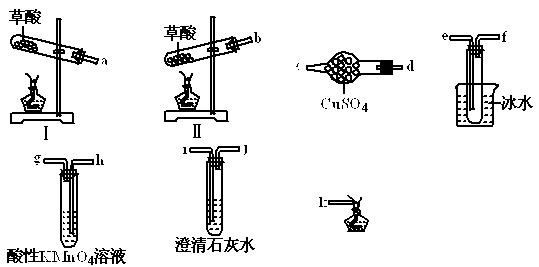
��1�����ᾧ�����ȷֽ�ķ���ʽΪ ��
��2�����ᾧ�����ȷֽ�ʱ��ѡ���װ��Ϊ �������� ��
��3����ֻ��֤CO2����һ�ֲ���ʱ�������巢��װ������ѡ��װ�õĽӿ�˳��Ϊ ��
��4������֤���еIJ���ʱ�������巢��װ������ѡ��ı�Ҫװ�õĽӿ�˳��Ϊ ��
��5�����ᾧ��ʹ���Ը��������Һ��ɫ�Ļ�ѧ����ʽΪ ��
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
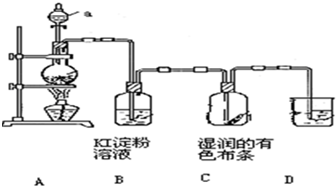 ij��ѧ�о���ѧϰС�������ȡ������̽�������ʵķ�����������ͼ��ʾװ�����ʵ�飮��A�з�����Ӧ�Ļ�ѧ����ʽΪ��MnO2+4HCl��Ũ���TMnCl2+
ij��ѧ�о���ѧϰС�������ȡ������̽�������ʵķ�����������ͼ��ʾװ�����ʵ�飮��A�з�����Ӧ�Ļ�ѧ����ʽΪ��MnO2+4HCl��Ũ���TMnCl2+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ������ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��2 L�ܱ������ڣ�800 ��ʱ��Ӧ��2NO(g)+O2(g) 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯���
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯��� ��
��
| ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
| n(NO)(mol) | 0.020 | 0.011 | 0.008 | 0.007 | 0.007 | 0.007 |
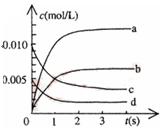
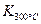 ��
��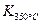 ��25 ��ʱ������1 mol NO2�������仯Ϊ56.4 kJ�����Ǹ�ͬѧ�������������ע����+������-����������Ŀ��Ϣ���÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��25 ��ʱ������1 mol NO2�������仯Ϊ56.4 kJ�����Ǹ�ͬѧ�������������ע����+������-����������Ŀ��Ϣ���÷�Ӧ���Ȼ�ѧ����ʽΪ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ӱ�ʡ��ɽһ�и����ڶ��ε��п������ۻ�ѧ���� ���ͣ������
ijͬѧ�������ϵ�֪���ٲ��ᾧ��(H2C2O4��2H2O)��175��ʱ���ȷֽ⣬ͬʱ�ۻ�������ʱ�ӷ����ڲ��ᾧ��������ˮ���������������ˮ��
Ϊ��֤����ֽ�IJ����ͬѧѡ�������������Լ��еIJ�����ɴ�ʵ�顣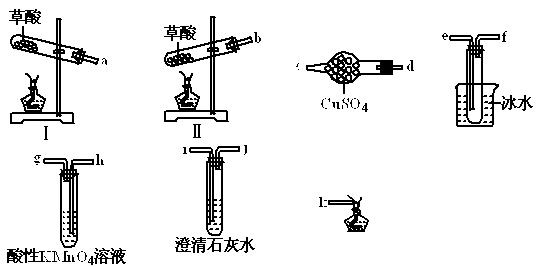
��1�����ᾧ�����ȷֽ�ķ���ʽΪ ��
��2�����ᾧ�����ȷֽ�ʱ��ѡ���װ��Ϊ �������� ��
��3����ֻ��֤CO2����һ�ֲ���ʱ�������巢��װ������ѡ��װ�õĽӿ�˳��Ϊ ��
��4������֤���еIJ���ʱ�������巢��װ������ѡ��ı�Ҫװ�õĽӿ�˳��Ϊ ��
��5�����ᾧ��ʹ���Ը��������Һ��ɫ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ӱ�ʡ�����ڶ��ε��п������ۻ�ѧ���� ���ͣ������
ijͬѧ�������ϵ�֪���ٲ��ᾧ��(H2C2O4��2H2O)��175��ʱ���ȷֽ⣬ͬʱ�ۻ�������ʱ�ӷ����ڲ��ᾧ��������ˮ���������������ˮ��
Ϊ��֤����ֽ�IJ����ͬѧѡ�������������Լ��еIJ�����ɴ�ʵ�顣
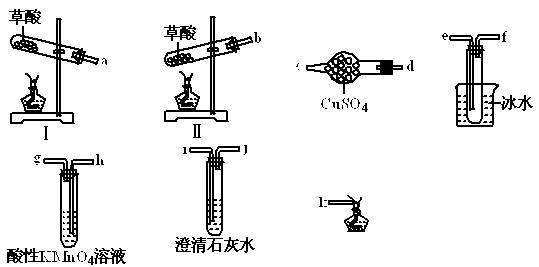
��1�����ᾧ�����ȷֽ�ķ���ʽΪ ��
��2�����ᾧ�����ȷֽ�ʱ��ѡ���װ��Ϊ �������� ��
��3����ֻ��֤CO2����һ�ֲ���ʱ�������巢��װ������ѡ��װ�õĽӿ�˳��Ϊ ��
��4������֤���еIJ���ʱ�������巢��װ������ѡ��ı�Ҫװ�õĽӿ�˳��Ϊ ��
��5�����ᾧ��ʹ���Ը��������Һ��ɫ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com