
����Ŀ��ij���dz��Ը���Ϊԭ�����ǣ�ͬʱ�õ������ĸ��������Ը����������ۺ����ò�����������ۺ�Ч�棬���һ��ܷ�ֹ������Ⱦ�������������£�

��֪ʯ���н��ѳ�Ϊ����H����Ҫ������E����Һ�ܷ���������Ӧ��G�Ǿ�����ζ��Һ�壬����ա�
��1��A������______��G������______��
��2��B�Ľṹ��ʽ_______��H�Ľṹ��ʽ____________��
��3��д��H�����Ӿ۷�Ӧ�ķ���ʽ��_____________��
��4��D��E�Ļ�ѧ����ʽ��_____________��
��5��E��F�Ļ�ѧ����ʽ��_____________��
��6��F��G�Ļ�ѧ����ʽ��_____________��
��7��д��G��ͬ���칹������CH3COOH��Ϊͬϵ��Ľṹ��ʽ��____________��
���𰸡� ��ά�� �������� CH2OH(CHOH)4CHO CH2=CH2 nCH2=CH2 ![]()
![]() 2CH3CH2OH+O2
2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O 2CH3CHO+ O2
2CH3CHO+2H2O 2CH3CHO+ O2 ![]() 2CH3COOH CH3COOH��CH3CH2OH
2CH3COOH CH3COOH��CH3CH2OH ![]() CH3COOCH2CH3+H2O CH3CH2CH2COOH��
CH3COOCH2CH3+H2O CH3CH2CH2COOH��![]()
�����������������������Ҫ�����л���Ľṹ�����ʡ�
��1�����ḻ��������ά�أ�����A����������ά�أ�D���Ҵ���F�����ᣬG���Ҵ����������ɵ���������G������������������
��2��B����ά�ص�ˮ����������ǣ�B�Ľṹ��ʽΪCH2OH(CHOH)4CHO��Hˮ�����Ҵ���H����ϩ��H�Ľṹ��ʽΪCH2=CH2��
��3��H�����Ӿ۷�Ӧ�Ļ�ѧ����ʽ��nCH2=CH2 ![]()
![]() ��
��
��4��D��E�Ҵ�������Ϊ��ȩ����ѧ����ʽ��2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��5��E��F��ȩ������Ϊ���ᣬ��ѧ����ʽ��2CH3CHO+ O2 ![]() 2CH3COOH��
2CH3COOH��
��6��F��G�Ҵ������ᷢ��������Ӧ����ѧ����ʽ��CH3COOH��CH3CH2OH ![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��7��G��ͬ���칹������CH3COOH��Ϊͬϵ����Ǻ���4��̼ԭ�ӵ����C3H7��COOH�����б���C3H7�������֣�CH3CH2CH2����(CH3)2CH�������Դ�Ϊ��CH3CH2CH2COOH��![]() ��
��


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�����ķ����У�����ԭ�Ӷ�����ͬһƽ�����
A. ���� B. �屽 C. �ױ� D. ������ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У���ԭ���������ǣ� ��
A. ��״����5.6L ���� B. 6.02��1022��H2SO4 C. 11gCO2 D. 4��ʱ9mLˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����ʵ�鱨�����г������������к�������( )
A. ��10mL��Ͳ��ȡ8.16mLϡ����
B. ��������ƽ����25.20g NaCl
C. �ù㷺pH��ֽ���ij��Һ��pHΪ2.3
D. ��25mL��ʽ�ζ��ܽ����к͵ζ�ʱ����ȥijŨ�ȵļ���Һ21.70mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
��1������Ԫ���е�һ��������С����_____________����Ԫ�ط��ţ���ͬ��
��2����Ѫ������A��B��C��D����Ԫ���γɵ���λ������C4[D(AB)6]��������ˮ���㷺����ʳ�����Ӽ��������������д����Ѫ�εĻ�ѧʽ___ _��1molABһ�к�����������ĿΪ__ ______(�����ӵ�������ֵΪNA)����Ѫ�ξ����и�����������������漰____ ������ţ���
a ���������� b�����ۼ� c����λ�� d�����Ӽ� e����� f�����Ӽ��������
��3��E2+�ļ۲�����Ų�ͼΪ ���ܶ�����л�����E���¿���H2�����ӳɷ�Ӧ����![]() ����̼ԭ�Ӳ�ȡsp2�ӻ��ķ����� ����������ţ���HCHO���ӵ�����ṹΪ �����ӳɺ����״����ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ���� ��
����̼ԭ�Ӳ�ȡsp2�ӻ��ķ����� ����������ţ���HCHO���ӵ�����ṹΪ �����ӳɺ����״����ۡ��е��CH4���ۡ��е�ߣ�����Ҫԭ���� ��
��4������C��F����ľ����ṹ��ͼ�������ж϶�Ӧ��ͼ����C��F���־��徧���н���ԭ�ӵ���λ��֮��Ϊ_ ___ ������C�ľ����У�����þ������ܶ�Ϊag/cm3�������ӵ�������ֵΪNA��Cԭ�ӵ�Ħ������ΪM g/mol�����ʾCԭ�Ӱ뾶�ļ���ʽΪ cm(���ػ���)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ұ����Խ��Խ�ܵ���ѧ�ҵ����ӣ�������Ĺؼ�֮һ��Ѱ��һЩ�����ø�������й������������(����)
A��ø��һ�ֵ�����
B��øֻ����ǿ���Ի������������²��ܷ�������
C��ø�Ĵ����и�Ч�ԡ�ѡ���Ժ�רһ��
D����չ����ұ���������ڽ�Լ��Դ�ͱ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���������ȷ���ǣ� ��
A. �ù��Ϊ10mL����Ͳ��ȡ6.20mL��Һ��
B. �����������ƹ��������ʱ���������������ֽ�ϣ�������������ƽ�����̣��������������ƽ������
C. ��������ɳ�ȥҺ̬������зе㲻ͬ���ӷ����ѻӷ��ӷ�������
D. �����Ȼ�̼��ȡ��ˮ�еĵ⣬��־��ú��Ϻ�ɫҺ�����ϲ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��Ӧ��2A��B![]() 2C������A��B��C��Ϊ���壬��ͼ�е������Ǹ÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�����ߣ�x���ʾ�¶ȣ�y���ʾB��ת���ʣ�ͼ����a��b��c���㣬������������ȷ����( )
2C������A��B��C��Ϊ���壬��ͼ�е������Ǹ÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�����ߣ�x���ʾ�¶ȣ�y���ʾB��ת���ʣ�ͼ����a��b��c���㣬������������ȷ����( )

A���÷�Ӧ�Ƿ��ȷ�Ӧ
B��b��ʱ��������ƽ��Ħ���������ٱ仯
C��T1�¶�������a��ﵽƽ�⣬���Բ�ȡ����ѹǿ�ķ���
D��c��v����v��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��úȼ���ŷŵ���������SO2��NOx���γ����ꡢ��Ⱦ����������NaClO2��Һ��Ϊ���ռ���ͬʱ���������������������ش��������⣺
��1�� NaClO2�Ļ�ѧ����Ϊ_______��
��2���ڹ��ݷ�Ӧ����ͨ�뺬��SO2��NO����������Ӧ�¶�Ϊ323 K��NaClO2��ҺŨ��Ϊ5��103 mol��L1 ����Ӧһ��ʱ�����Һ������Ũ�ȵķ���������±���
���� | SO42 | SO32 | NO3 | NO2 | Cl |
c/��mol��L1�� | 8.35��104 | 6.87��106 | 1.5��104 | 1.2��105 | 3.4��103 |
��д��NaClO2��Һ������������Ҫ��Ӧ�����ӷ���ʽ__________������ѹǿ��NO��ת����______�����ߡ��������䡱���͡�����
���������շ�Ӧ�Ľ��У����ռ���Һ��pH��______ ����������䡱��С������
����ʵ������֪������Ӧ����______������Ӧ���ʣ�����ڡ���С�ڡ�����ԭ���dz���SO2��NO�������еij�ʼŨ�Ȳ�ͬ����������___________��
��3���ڲ�ͬ�¶��£�NaClO2��Һ���������ķ�Ӧ�У�SO2��NO��ƽ���ѹpe��ͼ��ʾ��
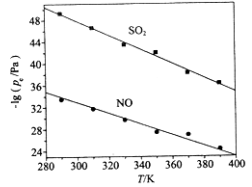
����ͼ������֪����Ӧ�¶����ߣ�����������Ӧ��ƽ�ⳣ����______________������������䡱��С������
�ڷ�ӦClO2+2SO32===2SO42+Cl��ƽ�ⳣ��K����ʽΪ___________��
��4���������NaClO��Ca��ClO��2���NaClO2��Ҳ�ܵõ��Ϻõ���������Ч����
�ٴӻ�ѧƽ��ԭ��������Ca��ClO��2���NaClO���е��ŵ���_______��
����֪���з�Ӧ��
SO2(g)+2OH (aq) ===SO32 (aq)+H2O(l) ��H1
ClO (aq)+SO32 (aq) ===SO42 (aq)+Cl (aq) ��H2
CaSO4(s) ===Ca2+��aq��+SO42��aq�� ��H3
��ӦSO2(g)+ Ca2+��aq��+ ClO (aq) +2OH (aq) === CaSO4(s) +H2O(l) +Cl (aq)�Ħ�H=______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com