
�������������ƷA��B����ɷֶ���(NH4)2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
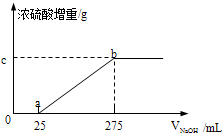
����ͬѧȡ��������ͬ��������ƷA����ˮ��Ȼ��ֱ���벻ͬ�����1mol/L��NaOH��Һ��ˮԡ����������ȫ���ݳ��������¶��£���β��ֽ⣩��������������������Ũ������ȫ���ա�Ũ�������ص����������NaOH��Һ������Ĺ�ϵ��ͼ��������ͼ���ش��������⣺

��1��д��ab���漰�����ӷ���ʽ�� ��
��2��c���Ӧ����ֵ�� ����ƷA��(NH4)2SO4��NH4HSO4�����ʵ���֮��Ϊ ������ͬѧȡ�����ݲ�ͬ��������ƷB���ֱ���뵽200mL 1mol/L��NaOH��Һ�У�ͬ����ˮԡ���ȣ����ݳ�������������ŨH2SO4���ա��ⶨ������±���
|
ʵ���� |
�� |
�� |
�� |
�� |
|
��������g�� |
9.88 |
19.76 |
29.64 |
49.40 |
|
Ũ�������ӵ�������g�� |
m |
m |
1.36 |
0 |
�����ñ������ش��������⣺
��3���ٷ���ʵ�����ݿ�֪��ʵ����Ϊ ��ʵ���У�������������������е�笠���
����ȫת�������壻m��ֵΪ ��
�ڼ�����ƷB�е�Ԫ�ص���������������С����ʾ��������λС����
��1������ͬѧ���о�ʱ���֣�Ũ�������ص���������ƷB������֮������һ���ĺ�����ϵ��������Ʒ������Ϊx(g)��Ũ�������ص�����Ϊy(g)����x�ڲ�ͬ��Χʱy��x�ĺ�����ϵ��
��1��NH4�� + OH�D = NH3��+ H2O (1��)
��2��4.25 (2��) 9��2 (3��)
��3���٢� (1��) 2.04 (2��) ��17% (3��)
��4��12.35<x<49.4�� y=3.4-17x/247 (4��)
��������
���������
��1��0-25ml: NaOH��Һ��NH4HSO4�е�H+���ã�2NH4HSO4+2NaOH= (NH4)2SO4+Na2 SO4+2H2O
ab�Σ�(NH4)2SO4+2NaOH=2 NH3��+ 2H2O+ Na2 SO4 ���ӷ���ʽ��NH4�� + OH�D = NH3��+ H2O
��2���ٽ⣺��Ũ�������ص�����Ϊx
(NH4)2SO4+2NaOH=2 NH3��+ 2H2O+ Na2 SO4
2 34
��275-25��/1000*1 x x=4.25 c���Ӧ����ֵ��4.25
����ƷA��(NH4)2SO4��NH4HSO4�����ʵ���֮��Ϊ9/2
�⣺��NH4HSO4�����ʵ���Ϊy
��2NH4HSO4+2NaOH= (NH4)2SO4+Na2 SO4+H2O
2 2 1
y 1*25/1000 0.025/2
y=0.025
�� (NH4)2SO4+2NaOH=2 NH3��+ 2H2O+ Na2 SO4
1 2
0.125 1*(275-25)/1000
ԭ(NH4)2SO4��0.125-0.0125=0.1125��mol��
(NH4)2SO4��NH4HSO4�����ʵ���֮��Ϊ0.1125/0.025=9/2
��3��I ��II��֪��I������٣�NaOH����������ܷ�Ӧ��֡�Ũ�������ص�����������������
�⣺��I����һ�ݣ�NH4HSO4��(NH4)2SO4�����ʵ���Ϊx��y��I����ζ���Ӧ��
115x+132y=9.88 �ų��İ��������ʵ���Ϊx+2y
��NH4HSO4 ---------2NaOH----- NH3�� ��2��(NH4)2SO4--------2NaOH-----2 NH3��
x x y 2y
�ڶ��ݵ������ǵ�һ�ݵ�2����NH4HSO4�����ʵ���ҲΪ2�������ʵ���Ϊ2x
���ڵڶ��ݣ���NH4HSO4---------H+ ---------OH-
2x 2x 2x
�ڶ������հ����������һ�ݵȣ����ʵ���Ҳ�ȡ�
NH4+---------OH-
x+2y x+2y ��NaOH��2x+ x+2y=0.2*1 ��115x+132y=9.88 x=0.04 y=0.04
�ų��İ��������ʵ���Ϊx+2y=0.12 �ų��İ���������Ϊ��m=17*0.12=2.04��g��
����ƷB�е�Ԫ�ص�����������14��x+2y��*100%/9.88=17%
60����I��֪��ƷB��NH4HSO4��(NH4)2SO4�����ʵ�����Ϊ0.04 mol�������ʵ���1:1��ϡ�
������Ʒ������Ϊx(g)��Ũ�������ص�����Ϊy(g)���м��������
��1��0.2 molNaOHǡ�÷�Ӧ��NH4HSO4��(NH4)2SO4������x(g)����NH4HSO4��(NH4)2SO4�����ʵ�����Ϊn
NH4HSO4-----------2NaOH ----------- NH3�� ��(NH4)2SO4-----------2NaOH----------- 2NH3��
n 2n n n 2n 2n
����NaOH ��4n=0.2 n=0.05 ��Ʒ������ x=115*0.05+132*0.05=12.35��g��
Ũ�������ص�����Ϊy��������������3n*17
��2��Ũ�������ص�����Ϊ0ʱ����Ʒ������Ϊx(g)����ʱNaOH �����㣬ֻ��H+��Ӧ
NH4HSO4- ----------NaOH
0��2 0.2
��ƷB�е�����Ϊx:0.2*115+0.2*132=49.4(g)
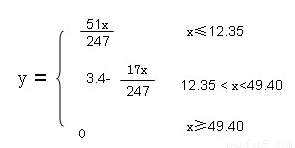
��x���ڵ���49.4 y=0 ��xС�ڵ���12.35 ��NH4HSO4��(NH4)2SO4���ʵ���1:1��ϣ����ʵ���Ϊx/247,������3 x/247������Ϊy=17*3 x/247=51x/247��12.35<x<49.4�� y=3.4-17x/247
���㣺������ʵ��Ϊ���������黯ѧ�����֪ʶ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
��2013?������һģ���������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
| ||
| ||
| ʵ���� | �� | �� | �� | �� |
| ��������g�� | 9.88 | 19.76 | 29.64 | 49.40 |
| Ũ�������ӵ�������g�� | m | m | 1.36 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����
�������������ƷA��B����ɷֶ��ǣ�NH4��2SO4��NH4HSO4�Ļ����ס��������о���ѧϰС���ͬѧ��Ҫȷ��A��B�и��ɷֵĺ�����| ʵ���� | �� | �� | �� | �� |
| ��������g�� | 9.88 | 19.76 | 29.64 | 49.40 |
| Ũ�������ӵ�������g�� | m | m | 1.36 | 0 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com