
(14��)�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ڽ���Һ����ͼ��ʾ������в�����

�ش��������⣺
(1)��ʼ��Һ��pH_____________7������ڡ�����С�ڡ����ڡ���ԭ����________________________________________��
(2)�Լ�I�Ļ�ѧʽΪ______________________�����з�����Ӧ�����ӷ���ʽΪ_________________________________________��
(3)�Լ���Ļ�ѧʽΪ______________________�����м����Լ����Ŀ����______________________________________��
(4)�Լ����������______________________�����з�����Ӧ�����ӷ���ʽΪ______________________________________��
(5)ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.7759g���ܽ������100mL����ƿ�У�ÿ��ȡ25.00mL��Һ����0.1000mol��L-1������������Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ25.62mL���ò�Ʒ�Ĵ���Ϊ ��
3�֣���1������ ̼����ᷢ��ˮ�⣬ʹ��Һ�Լ���
��3�֣���2��BaCl2��Һ Ba2++CO32-="==Ba" CO3��Ba2++SO42-="==Ba" SO4��
��3�֣� (3)K2CO3��Һ ��ȥ������BaCl2
��3�֣� (4)���� 2H++CO32-===H2O+CO2��
��2�֣�(5) 98.4%
��������
�����������1����ʼҺ���е��������Ȼ��ء�̼��ء�����أ���̼���Ϊǿ�������Σ��ᷢ��ˮ�⣬����Һ�ʼ��ԡ�
��2���Լ�I��Ҫ��ȥ���������Ӧ�������ӣ��ֿ��Dz�Ҫ����������������ʣ���ΪBaCl2��Һ ��
��3��A��Һ�к��е���Ҫ������Cl����K����Ba2������Ҫ��ȥ����ı����ӣ������ǹ����������ܼ����ܽ����ı���ȥ����ΪK2CO3��Һ��
��4���Լ�����Ϊ�˳�ȥ�����K2CO3����ѡ�����ᣬ���������ʡ�
��5��Ag����Cl��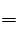 AgCl
��Ag����Ũ��Ϊ 0.02562L��0.1mol��L-1=2.562��10-3mol����Cl����Ũ��Ϊ 2.562��10-3mol����0.7759g��Ʒ��KCl������Ϊ
AgCl
��Ag����Ũ��Ϊ 0.02562L��0.1mol��L-1=2.562��10-3mol����Cl����Ũ��Ϊ 2.562��10-3mol����0.7759g��Ʒ��KCl������Ϊ
2.562��10-3mol��4��74.55g/mol=0.764g
�ʴ���Ϊ0.764��0.7759��100%=98.4%
���㣺���ӳ���
���������⿼�������ӵij��Ӽ���صļ��㣬�����ۺ��Ե���Ŀ��ѧ��Ҫ��Ϥ��Ԫ�ص�һ����ӷ��������ӵ�ԭ���Ѷ��еȡ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ��������Ӣ��ʵ��ѧУ������ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
(14��)�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ڽ���Һ����ͼ��ʾ������в�����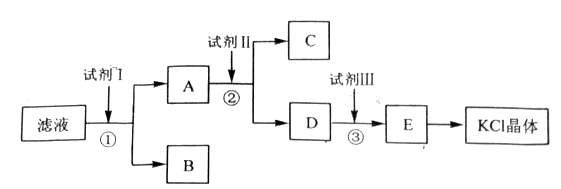
�ش��������⣺
(1)��ʼ��Һ��pH_____________7������ڡ�����С�ڡ����ڡ���ԭ����________________________________________��
(2)�Լ�I�Ļ�ѧʽΪ______________________�����з�����Ӧ�����ӷ���ʽΪ_________________________________________��
(3)�Լ���Ļ�ѧʽΪ______________________�����м����Լ����Ŀ����______________________________________��
(4)�Լ����������______________________�����з�����Ӧ�����ӷ���ʽΪ______________________________________��
(5)ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.7759g���ܽ������100mL����ƿ�У�ÿ��ȡ25.00mL��Һ����0.1000mol��L-1������������Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ25.62mL���ò�Ʒ�Ĵ���Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com