
(10��)�������������й㷺��Ӧ�ã�ij����������Ϊ��Ҫԭ���������ᡣ
��֪����550��ʱ��![]()
![]()
��һ���¶��£��������ڿ��������տ��ܷ������з�Ӧ(�������![]() ��
��![]() �����
�����
��Ϊ4��1)��![]() ,
,![]()
(1) 550��ʱ��6��4 g ![]() ������
������![]() ��ַ�Ӧ����
��ַ�Ӧ����![]() ���ų�����
���ų�����
9��83 kJ
(����ڡ��������ڡ���С�ڡ�)o
(2)��ʹ��Ӧ�ٵ�ƽ��������Ӧ�����ƶ������д�ʩ���е��� ��(����ĸ)
a����ƽ�������г���![]() b����ƽ�������г���
b����ƽ�������г���![]()
c���ı䷴Ӧ�Ĵ��� d�����ͷ�Ӧ���¶�
(3)Ϊʹ![]() ��ȫ����
��ȫ����![]() ������ʱҪʹ�ù�����
������ʱҪʹ�ù�����
 ��������������50��ʱ������¯����
��������������50��ʱ������¯����![]() ��
��
��������Ƕ���?
(4)720 g������![]() �ڿ�������ȫ���գ����ù���
�ڿ�������ȫ���գ����ù���
��![]() ��
��![]() �����ʵ���֮��n(
�����ʵ���֮��n(![]() )��n(
)��n(![]() )=6:
)=6: ![]() ��
��
��ʱ���Ŀ���Ϊ![]() mol��
mol��
����д������ڵĹ�ϵʽ�� ��
��������ͼ�л���![]() ��
��![]() �Ĺ�ϵ���ߡ�
�Ĺ�ϵ���ߡ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�����ص���ѧ��������ѧ�ȣ�������ѧ������������ѧ�Ծ����������� ���ͣ������
(6��) ����������������Ӧ�ù㷺��
(1)��ҵ�Ͽ���MnSO4��Һ�������������Mn2O3��Mn2O3�㷺Ӧ���ڵ��ӹ�ҵ��ӡȾ��ҵ��������д���û�ѧ��Ӧ�Ļ�ѧ����ʽ ��
(2)�����̲��ŷḻ���̽�˿�����Ҫ�ɷ���MnO2 ��1991����Allen�����о�����������ϴ��ʹ�ò�ͬ�ķ������Ʊ�������MnO2�����Ʊ���������ͼ��ʾ��
�ٲ���I�У��Լ��ױ�����е������� (�����)��
a. ������ b.��ԭ�� c.����
�ڲ�����У���NaClO3Ϊ��������������0.050 mol MnO2ʱ������0.10 mol��L��1��NaClO3��Һ200 mL ���÷�Ӧ�����ӷ���ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ��������ѧ�ȣ�������ѧ������������ѧ�Ծ��������棩 ���ͣ������
(6��) ����������������Ӧ�ù㷺��
(1)��ҵ�Ͽ���MnSO4��Һ�������������Mn2O3��Mn2O3�㷺Ӧ���ڵ��ӹ�ҵ��ӡȾ��ҵ��������д���û�ѧ��Ӧ�Ļ�ѧ����ʽ ��
(2)�����̲��ŷḻ���̽�˿�����Ҫ�ɷ���MnO2 ��1991����Allen�����о�����������ϴ��ʹ�ò�ͬ�ķ������Ʊ�������MnO2�����Ʊ���������ͼ��ʾ��
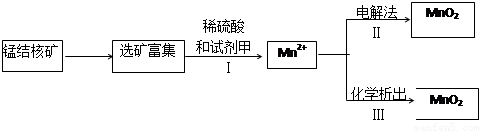
�ٲ���I�У��Լ��ױ�����е������� (�����)��
a. ������ b.��ԭ�� c.����
�ڲ�����У���NaClO3Ϊ��������������0.050 mol MnO2ʱ������0.10 mol��L��1 ��NaClO3��Һ200 mL ���÷�Ӧ�����ӷ���ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ������һ�ν�����Ͽ��Ի�ѧ�Ծ� ���ͣ������
(10��)����(CuSO4•5H20)�㷺���ڵ�ƹ��գ���ҽҩ���������������������ʹ��¼�����������ͭ����������������������ͼ��
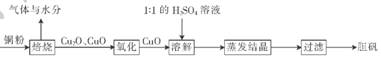
(1 )д���ܽ�����з�����Ӧ�����ӷ���ʽ��____________
(2)1 ��1��H2SO4��Һ����l���98%��H2SO4��1���ˮ��϶��ɡ����Ƹ���������Ĺ���������������������.����Ҫ______��______��
(3)��֪������ͭ(Cu2O)��ϡH2SO4��Ӧ��CuSO4��Cu���ɡ����豺�պ����ֻ��ͭ��������.Ϊ����ù���ijɷ�.����ʵ����ƺ�������__________________(����ĸ����
A. ����ϡH2SO4������Һ����ɫ��˵��������һ����CuO
B. ����ϡH2SO4�����к�ɫ���������ɣ�˵��������һ����Cu2O
C. ����ϡHNO3��������ɫ����(�漴��ɺ���ɫ��)������˵����������Cu2O
D. ����ϡHNO3��������ȫ���ܽ⣬˵��������û��Cu2O
(4)ȡ2.50g������Ʒ�������¼��ȷֽ⣬�ֽ���̵���������(��Ʒ�������¶ȱ仯������)��ͼ��ʾ��
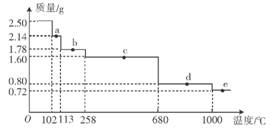
��a��ʱ�������ʵĻ�ѧʽΪ______��c��ʱ�������ʵĻ�ѧʽΪ______��
��10000Cʱ������Ӧ�Ļ�ѧ����ʽΪ_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(14��)���Ṥҵ��2SO2(g)��O2(g)������2SO3(g)����H<0(���ȷ�Ӧ)�й�ʵ���������£�
|
SO2�� ת���� �¶� |
1��105 Pa |
5��105 Pa |
10��105 Pa |
50��105 Pa |
100��105 Pa |
|
450 �� |
97.5% |
98.9% |
99.2% |
99.6% |
99.7% |
|
550 �� |
85.6% |
92.9% |
94.9% |
97.7% |
98.3% |
(1)�������г��ù����Ŀ�����Ϊ��________��
(2)���¶Ը÷�Ӧ�к�Ӱ�죿________��ʵ�������в���400��500 ����¶ȳ��˿������������⣬�����ǵ�________��
(3)����ѹǿ��������Ӧ�к�Ӱ�죿____������ҵ���ֳ����ó�ѹ���з�Ӧ����ԭ����______________��
(4)����ŨH2SO4������ˮ����SO3������___ ___��β����SO2������գ���Ҫ��Ϊ��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com