

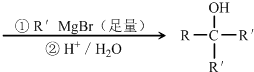




 +2NaOH
+2NaOH
 +NaBr+2H2O
+NaBr+2H2O +2NaOH
+2NaOH
 +NaBr+2H2O
+NaBr+2H2O

 ��ͨ��������Ӧ���е����۷�Ӧ���ɸ߾��
��ͨ��������Ӧ���е����۷�Ӧ���ɸ߾�� ��
�� ����E����������Ӧ����F��
����E����������Ӧ����F�� ���������ƴ���Һ�����������·�����ȥ��Ӧ��ͬʱ�����кͷ�Ӧ����
���������ƴ���Һ�����������·�����ȥ��Ӧ��ͬʱ�����кͷ�Ӧ���� ��
�� �ữ����E��EΪ
�ữ����E��EΪ �������Ȼ���C=C˫����FΪ
�������Ȼ���C=C˫����FΪ ������C=C˫����������G�к���C=C˫�������ǻ�����ѡ���Լ������Ȼ����������ǻ����ɣ������Ȼ����ǻ����Ʒ�Ӧ����������Ӧ��̼���������Ȼ���Ӧ�������Ʒ�Ӧ���ݴ˽�������
������C=C˫����������G�к���C=C˫�������ǻ�����ѡ���Լ������Ȼ����������ǻ����ɣ������Ȼ����ǻ����Ʒ�Ӧ����������Ӧ��̼���������Ȼ���Ӧ�������Ʒ�Ӧ���ݴ˽������� ��ͨ��������Ӧ���е����۷�Ӧ���ɸ߾���÷�Ӧ����ʽΪ
��ͨ��������Ӧ���е����۷�Ӧ���ɸ߾���÷�Ӧ����ʽΪ ��
�� ��
�� ���ʴ�Ϊ��C8H14O3��
���ʴ�Ϊ��C8H14O3�� ��
�� ������ȡ����Ӧ��
������ȡ����Ӧ�� ����E����������Ӧ����F��
����E����������Ӧ����F�� ���������ƴ���Һ�����������·�����ȥ��Ӧ��ͬʱ�����кͷ�Ӧ����
���������ƴ���Һ�����������·�����ȥ��Ӧ��ͬʱ�����кͷ�Ӧ���� ��C��D�ķ�Ӧ����ʽΪ
��C��D�ķ�Ӧ����ʽΪ +2NaOH
+2NaOH 
 +NaBr+2H2O��
+NaBr+2H2O�� +2NaOH
+2NaOH 
 +NaBr+2H2O��
+NaBr+2H2O�� �ữ����E��EΪ
�ữ����E��EΪ �������Ȼ���C=C˫����FΪ
�������Ȼ���C=C˫����FΪ ������C=C˫����������G�к���C=C˫�������ǻ��������Ȼ����ǻ����Ʒ�Ӧ����������Ӧ�������G����̼���������Ȼ���Ӧ�������Ʒ�Ӧ������E��F���ʴ�Ϊ��Na��NaHCO3��Һ��
������C=C˫����������G�к���C=C˫�������ǻ��������Ȼ����ǻ����Ʒ�Ӧ����������Ӧ�������G����̼���������Ȼ���Ӧ�������Ʒ�Ӧ������E��F���ʴ�Ϊ��Na��NaHCO3��Һ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com