
��Ȫ��һ�ֳ�������Ȼ���������ԭ���Ǵ���ѹǿ�
ͼ��Ϊ��ѧ��ѧ�г��õ���Ȫʵ��װ�á�����ƿ�г����������壬��ͷ�ιܼ��ձ��зֱ�ʢ��Һ�塣
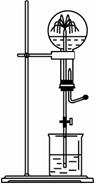
ͼ��
��1����������в������γ���Ȫ���ǣ�������
A��HCl��H2O B��O2��H2O
C��NH3��H2O D��CO2��NaOH��Һ
��2��ijѧ������˼��������Ȫ�������취�����������ͼ����ʾ��װ�á�
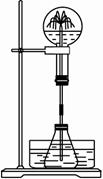
ͼ��
����ͼ�����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ����__________��
A��Cu��ϡ���� B��NaHCO3��NaOH��Һ
C��ͭ��ϡ���� D��NH4HCO3��ϡ����
����ͼ����ƿ���һˮ�ۣ�ƿ�м���ƾ���ˮ���м�����ˮ���ټ����������������ʣ����Ҳ��������Ȫ��ˮ���м�������ʿ�����__________��
A��Ũ���� B��ʳ�� C������� D������ͭ
���ַ���������Ȫ��ԭ����__________��
�۱Ƚ���ͼ����װ�ã��ò�����Ȫ��ԭ����������ͼ�����ϲ���ƿ������ѹǿ__________��ͼ�����²���ƿ������ѹǿ__________�����������С����
��3�������г�����������Ȫ����ɽ������ԭ��������__________���ͼ��ͼ��װ�õ�ԭ�����ơ�
��������1��С������ף�HCl��NH3��ˮ���ܽ�Ⱥܴ�CO2��NaOH��Һ���ܽ��Ҳ�ܴ�O2��ˮ���ܽ�Ⱥ�С��ѡB����2��С��٢���ڣ�1��С�����ƴ�����ͨ���γ�һ����ѹǿ�������Ȫ����֮ͬ���ǵڣ�1��С��ͨ����С��ƿ��ѹǿ�γ�ѹǿ�����С��٢���ͨ��������ƿ��ѹǿ�γ�ѹǿ���ѡ��C��D����ƿ��Ѹ�ٲ��������彫��Һѹ����ƿ�γ���Ȫ����ѡA����ΪŨ��������ˮ���ȣ��¶����ߣ��ƾ��ӷ��ӿ죬��ƿ��ѹǿ����2��С��۶��γ���Ȫ���ܵ�ԭ������˹������ܽᣬ��α�֪ʶ����ͼ���Ǽ�С��ƿ������ѹǿ��ͼ����������ƿ�ڵ�ѹǿ��
�𰸣���1��B ��2���� CD ��A��ΪŨ��������ˮ���ȣ��¶����ߣ��ƾ��ӷ��ӿ죬��ƿ��ѹǿ���� �ۼ�С ����3��ͼ��


 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Ȫ��һ�ֳ�������Ȼ����ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�ã���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ʾ��װ�ã���ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ���ǣ�������
��Ȫ��һ�ֳ�������Ȼ����ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�ã���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ʾ��װ�ã���ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��Ȫ��һ�ֳ�������Ȼ�����������ԭ���Ǵ���ѹǿ���ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����
��Ȫ��һ�ֳ�������Ȼ�����������ԭ���Ǵ���ѹǿ���ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ˮ | B��������̼������������Ʊ���Һ | C���Ȼ��������ˮ | D��һ������������������Ʊ���Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ȫ��һ�ֳ�������Ȼ���������ԭ���Ǵ���ѹǿ�
ͼ��Ϊ��ѧ��ѧ�г��õ���Ȫʵ��װ�á�����ƿ�г����������壬��ͷ�ιܼ��ձ��зֱ�ʢ��Һ�塣

ͼ��
��1����������в������γ���Ȫ���ǣ�������
A��HCl��H2O B��O2��H2O
C��NH3��H2O D��CO2��NaOH��Һ
��2��ijѧ������˼��������Ȫ�������취�����������ͼ����ʾ��װ�á�

ͼ��
����ͼ�����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ����__________��
A��Cu��ϡ���� B��NaHCO3��NaOH��Һ
C��ͭ��ϡ���� D��NH4HCO3��ϡ����
����ͼ����ƿ���һˮ�ۣ�ƿ�м���ƾ���ˮ���м�����ˮ���ټ����������������ʣ����Ҳ��������Ȫ��ˮ���м�������ʿ�����__________��
A��Ũ���� B��ʳ�� C������� D������ͭ
���ַ���������Ȫ��ԭ����__________��
�۱Ƚ���ͼ����װ�ã��ò�����Ȫ��ԭ����������ͼ�����ϲ���ƿ������ѹǿ__________��ͼ�����²���ƿ������ѹǿ__________�����������С����
��3�������г�����������Ȫ����ɽ������ԭ��������__________���ͼ��ͼ��װ�õ�ԭ�����ơ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com