��2010?��ͨģ�⣩���п�ͼ�漰������������Ԫ���У���һ��Ԫ���⣬�����Ϊ������Ԫ�أ���֪��A��EΪ�����嵥�ʣ�DΪ��ɫ�������ʣ�BΪ��ɫ��������ַ�Ӧ�IJ���δ��ȫ����
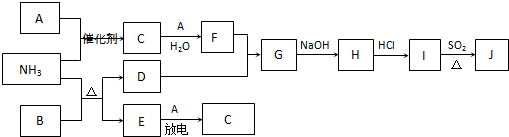
��ش��������⣺
��1����NH
3��F�����ʵ�����Ӧ�����Һ�У�����Ũ���ɴ�С��˳��Ϊ
c��NO3-����c��NH4+����c��H+����c��OH-��
c��NO3-����c��NH4+����c��H+����c��OH-��
��
��2��I��J��ͬ�ֽ������Ȼ����JΪ��ɫ��������SO
2��ԭI����J�����ӷ���ʽΪ
2Cu2++2Cl-+SO2+2H2O�T2CuCl��+4H++SO42-
2Cu2++2Cl-+SO2+2H2O�T2CuCl��+4H++SO42-
��
��3������β���г�����C��NH
3�ڼ��Ⱥʹ������ڵ�������������C����Ⱦ����֪��
��4NH
3��g��+5O
2��g��?4NO��g��+6H
2O��g����H=-905kJ?mol
-1 ��4NH
3��g��+3O
2��g��?2N
2��g��+6H
2O��g����H=-1268kJ?mol
-1��NH
3��C��Ӧ���Ȼ�ѧ����ʽΪ
6NO��g��+4NH3��g���T5N2��g��+6H2O��g����H=-1812.5 kJ?mol-1
6NO��g��+4NH3��g���T5N2��g��+6H2O��g����H=-1812.5 kJ?mol-1
��
��4��������Ϊ�������滯ѧ���о��ɹ���ʹNH
3��C�ķ�Ӧ�ڴ����������ʱ��Ч�ʴ����ߣ��Ӷ�ʹ��Ⱦ��D��ת���ʴ����ߣ�����Ӧ�û�ѧ�������۶Դ˹۵�������ۣ�
���о�ֻ�����ѧ��Ӧ���ʣ�����ʹ��ѧƽ�ⷢ���ƶ�
���о�ֻ�����ѧ��Ӧ���ʣ�����ʹ��ѧƽ�ⷢ���ƶ�
��





