
 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
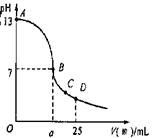
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
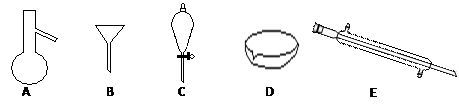
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������̼������Һ������ɫ�������ټ������ɫ������ʧ��˵����Ca2�� |
| B��ͨ������Cl2����Һ��Ϊ��ɫ���ټ��������Һ����Һ������˵����I�� |
| C���������������ʹ����ʯ��ˮ����ǵ����壬��ԭ��Һ��һ����CO32����SO32�� |
| D������Һ�м���BaCl2��Һ��ϡHNO3���а�ɫ�������ɣ�˵��һ����SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����еı��ӣ���ˮ�� | B���Ҵ��е�ˮ(���Ƶ�������) |
| C�������е����ᣨ����������Һ�� | D�����������е����ᣨ�Ҵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ϡ���� | B�� ϡ���� ϡ���� | C����ˮ | D������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ۢܢ� | B���ڢۢܢ�? | C���٢ۢݢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ӡ����軯�ء����ᡢ��������������Һ����FeCl3��Һһ�μ��� |
| B��CH3CH2OH��CH2=CHCOOH��CH3CHO��CH3COOH������Һ��������Cu(OH)2һ�μ��� |
| C����ȥKCl��Һ�е�����MgCl2������������NaOH��Һ������ |
| D��KCl��Һ�л�������KI����ͨ������Cl2�������Ҵ�������ȡ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
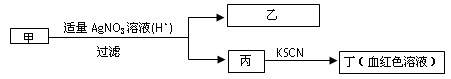
| A������һ����Fe3�� | B������һ����Fe3�� |
| C����ΪAgI���� | D����һ��ΪFeBr2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�����Ը��������Һ | B����������Һ������ | C������������Һ | D�������� |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com