
�����ڵ�һ��������⣬15������������ȼ�ϵ�ص���±ˮ (��Cl�D��Br�D��Na����Mg2��)��װ����ͼ��ʾ (a��bΪʯī�缫)������˵���У���ȷ����
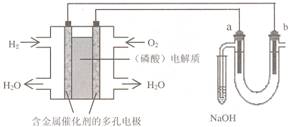
A����ع���ʱ��������ӦʽΪ��O2 ��2 H2O �� 4e��=4 OH�D
B�����ʱ��a �缫��Χ���ȷŵ����Br�D������Cl�D��˵��������������ͬʱǰ�ߵĻ�ԭ��ǿ�ں���
C�����ʱ����������·���ǣ����������·����������Һ������������
D������������ģ������������0.02g H2 ʱ��b ����Χ�����0.02g H2
BD
���⿼��ԭ��غ͵��أ��е��⡣��������Ϊ����ʣ���ص�������ӦΪ��O2 ��4H����4e����2H2O��A����a�缫��ԭ�������������Ϊ���ص�����������Br�D��ԭ��ǿ��Cl�D�����Br�D�ȷŵ磬B�ԣ����ʱ�����ʱ����������·���ǣ����������������������������Ӳ��ܴ���Һ��ͨ����C��������ͨ���ĵ����غ㣬�����������0.02g H2 ʱ��b ����Χ�����0.02g H2��D�ԡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com