
ijѧϰС�����о���
Fe2+�Ʊ�Fe(OH)2�Ĺ��������������ʵ�鷽����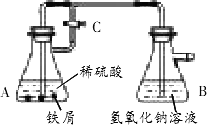
������ͼ����ʵ�飬����
A�еķ�Ӧ��ʼʱ������C���ڴ�״̬��һ��ʱ��رջ���C������A�еķ�Ӧ���ڽ��У�ʵ���г��ֵİ�ɫ��������Ϊ����ɫ����ɫ����ν��ʹ������أ���ͬѧ�������Ϻõ�
Fe(OH)2��������Ϣ����ɫ����ǿ������O2��Ѹ�ٱ�ɺ��ɫFe(OH)3����ʱ��Ϊ�������̵��м���Ϊ����ɫ�����к���Fe2+��Fe3+���ش��������⣺
(1)�Ķ��������ϣ���Ը�ʵ���г��ֻ���ɫ����ɫ�Ľ�����________��
(2)��ͬѧ������ɫԭ������ɫ����ɫ�����ܵ������ɫ�����ɫ����Ϊ��ɫ������Fe(OH)2��nH2O���£�����ˮԡ�������ɵ���ɫ�������۲쵽���������̱�����ƣ�����ʱ������CӦ����________(����رա�)״̬������A�еķ�Ӧ�账��________(�ֹͣ��������)״̬��д����ʵ����֧����ͬѧ�۵�Ļ�ѧ����ʽ��________��
(3)�����һ�����о��Ʊ��Ĺ����������ڶԳ����ɰױ���������к������͵�ʵ�������˼·��________��
|
�����𰸣� (1)Fe(OH)2���ֱ������������������ɫ��Fe(OH)3���ڰ�ɫ��Fe(OH)2������ (2)��������Fe(OH)2��nH2O���� (3)��Fe2+��Fe3+�Ļ����Һ�м���NaOH��Һ�۲����ɳ�������ɫ�Ƿ�Ϊ����ɫ[���߿��Խ�Fe(OH)2��Fe(OH)3��Ϻ�۲�����ɫ]������ȷ�ⷨ�� (1)�����Ͽ�֪Fe(OH)2�ֱ�����������������Fe(OH)3����ʾ����ɫ������ (2)Ϊ�˷�ֹFe(OH)2������������B�е���ɫ����ʱ��A�еķ�ӦҪ���ڷ���״̬��ͬʱ����C��ʹB�еķ�Ӧ��������Χ�н��У�B�з���֧����ͬѧ�۵�ķ�Ӧ��Fe(OH)2��nH2O�ֽ⣮���� (3)�����ɰױ��̵���������Fe2+��Fe3+�Ļ����Һ�м���NaOH��Һ���۲����ɳ�������ɫ�Ƿ�Ϊ����ɫ�����������������ԭ���ǽ̲����������������Ʊ�ʵ�� ,�����������������IJ��ȶ��ԣ�������������������������ɫ�ȣ� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʵ �� �����Һ |
A | B | C | D | E | F |
| 4mol?L-1H2SO4/mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ/mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O/mL | V7 | V8 | V9 | 15 | 10 | 0 |
| ��� | c��HA��/mol?L-1 | c��NaOH��/mol?L-1 | ���ҺpH |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�������ϡ������п��ȡ������ʵ���У�������������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
��1��������������ͭ��Һ���Լӿ������������ʵ�ԭ���� ��
��2��Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ�� �������֣���
��3��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬ijѧϰС�����������һϵ��ʵ�顣�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
| ʵ �� �����Һ | A | B | C | D | E | F |
| 4 mol��L-1H2SO4 / mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ / mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O / mL | V7 | V8 | V9 | 15 | 10 | 0 |
����ɴ�ʵ����ƣ����У�V2 V5�� �� V6�� ��V8�� ��
����һ����������������������������������ֵ��
�����£�ijһԪ��HA��NaOH��Һ�������ϣ�HA��NaOH��Ũ���Լ���Ϻ���Һ��pH���±���
| ��� | c��HA��/mo1��L-1 | c��NaOH��/mo1��L-1 | ���ҺpH |
| �� | c | 0.2 | pH = 7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH = 9 |
��ش��������⣺
��4�����Ӽ������������c�Ƿ�һ������0.2 �� ��ѡ��ǡ�����
��5����������ʵ�����ݣ�HA�� �ᣨѡ�ǿ�������������û��Һ������Ũ���ɴ�С��˳���� ��
��6���������û��Һ����ˮ�������c(OH-) = mo1��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�������ϡ������п��ȡ������ʵ���У�������������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
��1��������������ͭ��Һ���Լӿ������������ʵ�ԭ���� ��
��2��Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ�� �������֣���
��3��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬ijѧϰС�����������һϵ��ʵ�顣�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
| ʵ �� �����Һ | A | B | C | D | E | F |
| 4 mol��L-1H2SO4 / mL | 30 | V1 | V2 | V3 | V4 | V5 |
| ����CuSO4��Һ / mL | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
| H2O / mL | V7 | V8 | V9 | 15 | 10 | 0 |
����ɴ�ʵ����ƣ����У�V2 V5 �� �� V6�� ��V8�� ��
����һ����������������������������������ֵ��
�����£�ijһԪ��HA��NaOH��Һ�������ϣ�HA��NaOH��Ũ���Լ���Ϻ���Һ��pH���±���
| ��� | c��HA��/mo1��L-1 | c��NaOH��/mo1��L-1 | ���ҺpH |
| �� | c | 0.2 | pH = 7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH = 9 |
��ش��������⣺
��4�����Ӽ������������c�Ƿ�һ������0.2 �� ��ѡ��ǡ�����
��5����������ʵ�����ݣ�HA�� �ᣨѡ�ǿ�������������û��Һ������Ũ���ɴ�С��˳���� ��
��6���������û��Һ����ˮ�������c(OH-) = mo1��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ�߶���һѧ���������⿼�Ի�ѧ�Ծ� ���ͣ������
��14�֣�������ϡ������п��ȡ������ʵ���У�������������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
��1��������������ͭ��Һ���Լӿ������������ʵ�ԭ���� ��
��2��Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ�� �������֣���
��3��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬ijѧϰС�����������һϵ��ʵ�顣�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
|
ʵ �� �����Һ |
A |
B |
C |
D |
E |
F |
|
4 mol��L-1H2SO4 / mL |
30 |
V1 |
V2 |
V3 |
V4 |
V5 |
|
����CuSO4��Һ / mL |
0 |
0.5 |
2.5 |
5 |
V6 |
20 |
|
H2O / mL |
V7 |
V8 |
V9 |
15 |
10 |
0 |
����ɴ�ʵ����ƣ����У�V2 V5 �� �� V6�� ��V8�� ��
����һ����������������������������������ֵ��
�����£�ijһԪ��HA��NaOH��Һ�������ϣ�HA��NaOH��Ũ���Լ���Ϻ���Һ��pH���±���
|
��� |
c��HA��/mo1��L-1 |
c��NaOH��/mo1��L-1 |
���ҺpH |
|
�� |
c |
0.2 |
pH = 7 |
|
�� |
0.2 |
0.1 |
pH��7 |
|
�� |
0.1 |
0.1 |
pH = 9 |
��ش��������⣺
��4�����Ӽ������������c�Ƿ�һ������0.2 �� ��ѡ��ǡ�����
��5����������ʵ�����ݣ�HA�� �ᣨѡ�ǿ�������������û��Һ������Ũ���ɴ�С��˳���� ��
��6���������û��Һ����ˮ�������c(OH-) = mo1��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�츣��ʡ�����и߶���ѧ���������⿼�Ի�ѧ�Ծ� ���ͣ������
��14�֣�������ϡ������п��ȡ������ʵ���У�������������ͭ��Һ�ɼӿ��������������ʡ���ش��������⣺
��1��������������ͭ��Һ���Լӿ������������ʵ�ԭ���� ��
��2��Ҫ�ӿ�����ʵ����������������ʣ����ɲ�ȡ�Ĵ�ʩ�� �������֣���
��3��Ϊ�˽�һ���о�����ͭ�����������������ʵ�Ӱ�죬ijѧϰС�����������һϵ��ʵ�顣�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���ķ�Ӧƿ�У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
|
ʵ �� �����Һ |
A |
B |
C |
D |
E |
F |
|
4 mol��L-1H2SO4 / mL |
30 |
V1 |
V2 |
V3 |
V4 |
V5 |
|
����CuSO4��Һ / mL |
0 |
0.5 |
2.5 |
5 |
V6 |
20 |
|
H2O / mL |
V7 |
V8 |
V9 |
15 |
10 |
0 |
����ɴ�ʵ����ƣ����У�V2 V5 �� �� V6�� ��V8�� ��
����һ����������������������������������ֵ��
�����£�ijһԪ��HA��NaOH��Һ�������ϣ�HA��NaOH��Ũ���Լ���Ϻ���Һ��pH���±���
|
��� |
c��HA��/mo1��L-1 |
c��NaOH��/mo1��L-1 |
���ҺpH |
|
�� |
c |
0.2 |
pH = 7 |
|
�� |
0.2 |
0.1 |
pH��7 |
|
�� |
0.1 |
0.1 |
pH = 9 |
��ش��������⣺
��4�����Ӽ������������c�Ƿ�һ������0.2 �� ��ѡ��ǡ�����
��5����������ʵ�����ݣ�HA�� �ᣨѡ�ǿ�������������û��Һ������Ũ���ɴ�С��˳���� ��
��6���������û��Һ����ˮ�������c(OH-) = mo1��L-1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com