��2012?������һģ��ij��ѧС�����������װ�ã��гֺͼ�����������ȥ������ⱥ��ʳ��ˮ�����õ�������H
2��ԭCuO��ĩ���ⶨCu�����ԭ��������ͬʱ���������������ԣ�
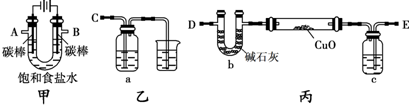
��1��д����װ���е�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ
2NaCl+2H
2O
2NaOH+H
2��+Cl
2��
2NaCl+2H
2O
2NaOH+H
2��+Cl
2��
��
��2��Ϊ�������ʵ�飬��ȷ������˳��ΪA��
E
E
��B��
C
C
����д���ܿ���ĸ����
��3����Ӳ�ʲ������������ͭ��ĩ����ǰ����Ҫ���еIJ���Ϊ
��ͨһ��ʱ����������D�����������Ĵ���
��ͨһ��ʱ����������D�����������Ĵ���
��
��4�������������������ԣ�����װ�õ�aƿ����Һ������
����KI��Һ
����KI��Һ
����Ӧ������Ϊ
��Һ��Ϊ��ɫ
��Һ��Ϊ��ɫ
����װ���ձ��з�����Ӧ�����ӷ���ʽ��
Cl2+2OH-=Cl-+ClO-+H2O
Cl2+2OH-=Cl-+ClO-+H2O
��
��5����װ�õ�cƿ��ʢ�ŵ��Լ�Ϊ
Ũ����
Ũ����
��������
����H2�е�H2O����ֹӲ�ʲ�����ը�ѣ���Ӱ��ⶨˮ������
����H2�е�H2O����ֹӲ�ʲ�����ը�ѣ���Ӱ��ⶨˮ������
��
��6��Ϊ�ⶨCu�����ԭ����������������¼ס�������ʵ�鷽������ȷ����Ӳ�ʲ����ܵ�����Ϊag������CuO��ȷ����Ӳ�ʲ����ܺ�CuO��������Ϊbg������CuO��ַ�Ӧ����ʵ����Ϻ�
������ͨ����ȷ����Ӳ�ʲ����ܺ�Cu�۵�������Ϊc g������ȷ��Cu�����ԭ��������
�ҷ�����ͨ����ȷ�ⶨ����ˮ������d g������ȷ��Cu�����ԭ��������
������������ش�
��
��
������������ȷ������Ϊ�������ķ����IJ���֮����
�����е�CO2��H2Oͨ��D�ڽ���U�ι����ʵ�����ϴ�
�����е�CO2��H2Oͨ��D�ڽ���U�ι����ʵ�����ϴ�
��
���������������ⶨ�����ݼ��㣬Cu�����ԭ������Ϊ
��





 ij��ѧС�����������װ�ã��гֺͼ�����������ȥ������ⱥ��ʳ��ˮ�����õ�������H2��ԭCuO��ĩ���ⶨCu�����ԭ��������ͬʱ���������������ԣ�
ij��ѧС�����������װ�ã��гֺͼ�����������ȥ������ⱥ��ʳ��ˮ�����õ�������H2��ԭCuO��ĩ���ⶨCu�����ԭ��������ͬʱ���������������ԣ�



