
![]() ��һ�ִ�����Ⱦ�ij��ȤС����̽��
��һ�ִ�����Ⱦ�ij��ȤС����̽��![]() �����ʼ���ɫʵ��ķ�����������·�����
�����ʼ���ɫʵ��ķ�����������·�����
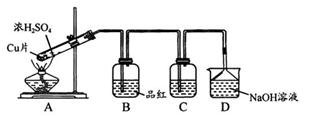
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
Ϊ��ʵ����ɫʵ���Ŀ�꣬��ͬѧ�������������ͼA����ȡװ�ã�
��Aװ����ȣ�A�� װ�õ��ŵ��ǣ�
�� ��
�� ��
(2)����A�� װ�ú�����������ʵ�顣
I����֤![]() �������ԣ�Cװ���п�ѡ�Լ� (�����)��
�������ԣ�Cװ���п�ѡ�Լ� (�����)��
A��Ba(HCO3)2��Һ B�������� C����ˮ D��Ʒ����Һ
II����֤![]() �Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�ø��Լ��������Ǣ� ���� ��
(3)Dװ���е����¶˵���©���������� ��
(4)��ҵ���������������Σ��������û������Ʊ�![]() �Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
�Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
��β���ð����յ�Ŀ���ǣ� ��w^w

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡ1mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�����Һ�д���SO32-�� SO42-�� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� ����1�Σ���������Ʒ�� ����1�Σ���������Ʒ�� �ٵ������2mol/L���ᣬ�� �ٵ������2mol/L���ᣬ�� �� |
��Ʒ����ɫ���������ݣ���������Һ���д��� SO32-�� ��Ʒ����ɫ���������ݣ���������Һ���д��� SO32-�� ��Ʒ�첻��ɫ�����������ݣ���������Һ���в����� SO32- ��Ʒ�첻��ɫ�����������ݣ���������Һ���в����� SO32- |
| ����3�� ���Թ�ȡ������Һ ���Թ�ȡ������Һ �����е��������1mol/LBa��OH��2��Һ[�����1�Σ���������Ʒ����Һ���ٵ���2-3�Σ����������2mol/L����]���� �����е��������1mol/LBa��OH��2��Һ[�����1�Σ���������Ʒ����Һ���ٵ���2-3�Σ����������2mol/L����]���� �� |
�����ְ�ɫ��������Ʒ����Һ��ɫ���������ݣ���������Һ���д��� HSO3-�� �����ְ�ɫ��������Ʒ����Һ��ɫ���������ݣ���������Һ���д��� HSO3-�� �������ְ�ɫ��������Ʒ����Һ����ɫ����û�����ݣ���������Һ���в����� HSO3-�� �������ְ�ɫ��������Ʒ����Һ����ɫ����û�����ݣ���������Һ���в����� HSO3-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��)![]() ��һ�ִ�����Ⱦ�ij��ȤС����̽��
��һ�ִ�����Ⱦ�ij��ȤС����̽��![]() �����ʼ���ɫʵ��ķ�����������·�����
�����ʼ���ɫʵ��ķ�����������·�����
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
Ϊ��ʵ����ɫʵ���Ŀ�꣬��ͬѧ�������������ͼA����ȡװ�ã�
��Aװ����ȣ�A�� װ�õ��ŵ��ǣ�
�� ��
�� ��
(2)����A�� װ�ú�����������ʵ�顣
I����֤![]() �������ԣ�Cװ���п�ѡ�Լ� (�����)��
�������ԣ�Cװ���п�ѡ�Լ� (�����)��
A��Ba(HCO3)2��Һ B�������� C����ˮ D��Ʒ����Һ
II����֤![]() �Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�ø��Լ��������Ǣ� ���� ��
(3)Dװ���е����¶˵���©���������� ��
(4)��ҵ���������������Σ��������û������Ʊ�![]() �Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
�Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
��β���ð����յ�Ŀ���ǣ� ��w^w
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����㶫ʡ���ڸ���ѧ������ѧ�����п��ԣ����ۣ���ѧ���� ���ͣ�ʵ����
(14��) ��һ�ִ�����Ⱦ�ij��ȤС����̽��
��һ�ִ�����Ⱦ�ij��ȤС����̽�� �����ʼ���ɫʵ��ķ�����������·�����
�����ʼ���ɫʵ��ķ�����������·�����
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
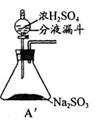
Ϊ��ʵ����ɫʵ���Ŀ�꣬��ͬѧ�������������ͼA����ȡװ�ã�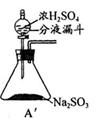
��Aװ����ȣ�A�� װ�õ��ŵ��ǣ�
�� ��
�� ��
(2)����A�� װ�ú�����������ʵ�顣
 I����֤
I����֤ �������ԣ�Cװ���п�ѡ�Լ� (�����)��
�������ԣ�Cװ���п�ѡ�Լ� (�����)��
| A��Ba(HCO3)2��Һ | B�������� | C����ˮ | D��Ʒ����Һ |
 �Ļ�ԭ�ԣ�Cװ���п����Լ� (������)��
�Ļ�ԭ�ԣ�Cװ���п����Լ� (������)�� �Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________��
�Ļ�ѧ��Ӧ����ʽΪ��___________________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��У������һ��������ѧ�Ծ��������棩 ���ͣ�ʵ����
��10�֣�SO2��һ�ִ�����Ⱦ�ij��ȤС����̽��SO2�����ʼ���ɫʵ�鷽����������·�����

�� B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԡ�����C��D�ֱ�Ϊ��ˮ�������ˮ��Һ����B����ʢ�Լ�Ϊ______________��C�з�Ӧ�����ӷ���ʽΪ��____________________________________________��
�� Ϊ��ʵ����ɫʵ���Ŀ�꣬ijͬѧ���������������ͼA2����ȡװ��������A1װ�ã���A1װ����ȣ�A2װ�õ��ŵ��ǣ�____________________________________________________����дһ�㼴�ɣ���
�� E���ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH����SO32����SO42����HSO3���������ӡ���֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2mol/L���ᡢ2mol/L HNO3��1mol/L BaCl2��Һ��1mol/L Ba(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32����HSO3��������±���ʵ�������Ԥ������ͽ��ۣ�
|
ʵ����� |
Ԥ����������� |
|
����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡ1mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� |
�����ְ�ɫ���ǣ�����Һ�д���SO32���� SO42���� |
|
����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ���_______________ _______________________________________________________ |
_________________________ ____________________________ |
|
����3______________________________________________ ____________________________________________________ |
_________________________ _________________________ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com