
��C6H10O5��n+nH2O![]() nC6H12O6
nC6H12O6
���� ������
C6H12O6![]() 2C2H5OH+2CO2��
2C2H5OH+2CO2��
������
��C6H10O5��n����nC6H12O6����2nC2H5OH
����ƤΪԭ���÷��ͷ���ȡʳ�ף�����1 000 kg������20%����Ƥ���ڷ���������80%�ĵ���ת��Ϊ�Ҵ������Ҵ�����������IJ���Ϊ90%�������տ��Ƶ�5%��ʳ����ǧ�ˣ�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ѧϰ�ܱ�����ѧ���˽̿α�߶���(ѡ��4)��2009��2010ѧ�ꡡ��2�ڡ��ܵ�158�� �˽̿α��(ѡ��4) ���ͣ�022
�����Ҵ����ɵ��ۻ���ά�ص�������ԭ�Ϸ��ͻ�ã������Ҵ�����ȡ������Ҳ�����Ҵ�������صĻ���������
(1)��֪���Ҵ���ȡ��������������·�ߣ�
a��ˮ������������
CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g)
b�����ִ�������
CH3CH2OH(g)��![]() O2(g)��3H2(g)��2CO(g)
O2(g)��3H2(g)��2CO(g)
ԭ�������ʽϸߵ���
________(ѡ�a����b��)ʽ��(2)��֪CO��ȼ����Ϊ283 kJ/mol��H2��ȼ����Ϊ285.8 kJ/mol����ȡ2 mol�Ҵ���40����aʽ���⣬60����bʽ���⣬�ٶ�ת����Ϊ100�������������������ȫ��������ȫȼ�ղ���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�콭��������ѧ������ǰ����ѵ����ѧ���� ���ͣ�������
ˮú������Ҫȼ�Ϻͻ���ԭ�ϣ�����ˮ����ͨ�����ȵ�̿���Ƶã�
C (s) + H2O(g) CO (g) +H2 (g) ��H�� +131.3 kJ?mol��1����������������
CO (g) +H2 (g) ��H�� +131.3 kJ?mol��1����������������
��1��ʵ�ʹ�ҵ�����У���̿��������ͨ��ˮ�����Ϳ���������ͨ�������ԭ�������ڸ÷�Ӧ�����ȣ�����̿���¶Ƚ��ͣ��뼰ʱͨ�븻�������ٽ�̿���ȼ�շ��ȣ�
C (s) + O2(g)= CO2 (g)����H = ��393.5kJ��mo1��1 ��������������
Ϊ���������������ԣ������������������IJ�������ģ���ÿ���Ӧͨ���ˮ�����Ϳ���������ȣ�ͬ��ͬѹ��ԼΪ���٣�������������������ռ1/5��
��2��һ���¶��£����������о�������������Ӧ�٣���������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д������Ӧ�Ŀո�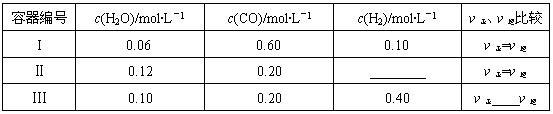
��3�������Ҵ����ɵ��ۻ���ά�ص�������ԭ�Ϸ��ͻ�á������Ҵ��ɽ�����úϳ�����CO��H2�������Ҵ������ϳ�������������·�ߣ�
a��ˮ������������CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g)
b�����ִ�������CH3CH2OH(g)��1/2O2(g)��3H2(g)��2CO(g)
ij���������о����������Ҵ��õ��ĺϳ����ϳ�һ���������͡��Ҵ�����һ�밴a��b��ʽ��Ӧ���ϳ����ϳ��������͵ķ�ӦΪ��2mCO��(2m��n)H2��2CmHn��2mH2O���ٶ��ϳɵ����������к���X��Y���ֳɷ֣���X��Y������8��̼ԭ�ӵ�����X�DZ���ͬϵ�Y��������
��X�ķ���ʽΪ ��Y�ķ���ʽΪ ��
��50����������Ϊ92%���Ҵ�������ת�����ٶ�����ת���ʾ�Ϊ100%���������տɻ��X������Ϊ���ٶ֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮú������Ҫ
ȼ�Ϻͻ���ԭ�ϣ�����ˮ����ͨ�����ȵ�̿���Ƶã�
C (s) + H2O(g) ![]() CO (g) +H2 (g) ��H�� +131.3 kJ•mol��1
CO (g) +H2 (g) ��H�� +131.3 kJ•mol��1
��1��ʵ�ʹ�ҵ�����У���̿��������ͨ��ˮ�����Ϳ�������ԭ���� ��
��2��һ���¶��£����������о�������������Ӧ����������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д������Ӧ�Ŀո�
| ������� | c(H2O)/mol��L��1 | c(CO)/mol��L��1 | c(H2)/mol��L��1 | �����������Ƚ� |
| I | 0.06 | 0.60 | 0.10 | ����=���� |
| �� | 0.12 | 0.20 | ________ | ����=���� |
| �� | 0.10 | 0.20 | 0.40 | ����____���� |
��3�������Ҵ����ɵ��ۻ���ά�ص�������ԭ�Ϸ��ͻ�á������Ҵ��ɽ�����úϳ�����CO��H2����
���Ҵ������ϳ�������������·�ߣ�
a��ˮ������������CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g)
b�����ִ�������CH3CH2OH(g)��1/2O2(g)��3H2(g)��2CO(g)
ij���������о����������Ҵ��õ��ĺϳ����ϳ�һ���������͡��Ҵ�����һ�밴a��b��ʽ��Ӧ���ϳ����ϳ��������͵ķ�ӦΪ��2mCO��(2m��n)H2��2CmHn��2mH2O���ٶ��ϳɵ����������к���X��Y���ֳɷ֣���X��Y������8��̼ԭ�ӵ�����X�DZ���ͬϵ�Y��������
��X�ķ���ʽΪ ��Y�ķ���ʽΪ ��
��50����������Ϊ92%���Ҵ�������ת�����ٶ�����ת���ʾ�Ϊ100%���������տɻ��X������Ϊ���ٶ֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ˮú������Ҫȼ�Ϻͻ���ԭ�ϣ�����ˮ����ͨ�����ȵ�̿���Ƶã�
C (s) + H2O(g) ![]() CO (g) +H2 (g) ��H�� +131.3 kJ??mol��1����������������
CO (g) +H2 (g) ��H�� +131.3 kJ??mol��1����������������
��1��ʵ�ʹ�ҵ�����У���̿��������ͨ��ˮ�����Ϳ���������ͨ�������ԭ�������ڸ÷�Ӧ�����ȣ�����̿���¶Ƚ��ͣ��뼰ʱͨ�븻�������ٽ�̿���ȼ�շ��ȣ�
C (s) + O2(g)= CO2 (g)����H = ��393.5kJ��mo1��1 ��������������
Ϊ���������������ԣ������������������IJ�������ģ���ÿ���Ӧͨ���ˮ�����Ϳ���������ȣ�ͬ��ͬѹ��ԼΪ���٣�������������������ռ1/5��
��2��һ���¶��£����������о�������������Ӧ�٣���������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д������Ӧ�Ŀո�

��3�������Ҵ����ɵ��ۻ���ά�ص�������ԭ�Ϸ��ͻ�á������Ҵ��ɽ�����úϳ�����CO��H2�������Ҵ������ϳ�������������·�ߣ�
a��ˮ������������CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g)
b�����ִ�������CH3CH2OH(g)��1/2O2(g)��3H2(g)��2CO(g)
ij���������о����������Ҵ��õ��ĺϳ����ϳ�һ���������͡��Ҵ�����һ�밴a��b��ʽ��Ӧ���ϳ����ϳ��������͵ķ�ӦΪ��2mCO��(2m��n)H2��2CmHn��2mH2O���ٶ��ϳɵ����������к���X��Y���ֳɷ֣���X��Y������8��̼ԭ�ӵ�����X�DZ���ͬϵ�Y��������
��X�ķ���ʽΪ ��Y�ķ���ʽΪ ��
��50����������Ϊ92%���Ҵ�������ת�����ٶ�����ת���ʾ�Ϊ100%���������տɻ��X������Ϊ���ٶ֣�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com