
| ���� | ���Ӱ뾶��pm�� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 74 | 7.6 | 9.7 |
| Fe3+ | 64 | 2.7 | 3.7 |
| Al3+ | 50 | 3.8 | 4.7 |
| Mn2+ | 80 | 8.3 | 9.8 |
| Pb2+ | 121 | 8.0 | 8.8 |
| Ca2+ | 99 | - | - |
 ��֪PbO2�������Դ���MnO2����ش��������⣺
��֪PbO2�������Դ���MnO2����ش��������⣺

 �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ���� | ���Ӱ뾶��pm�� | ��ʼ����pH | ��ȫ����pH |
| Fe2+ | 74 | 7.6 | 9.7 |
| Fe3+ | 64 | 2.7 | 3.7 |
| Al3+ | 50 | 3.8 | 4.7 |
| Mn2+ | 80 | 8.3 | 9.8 |
| Pb2+ | 121 | 8.0 | 8.8 |
| Ca2+ | 99 | - | - |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����SO2���ŷš���������SO2��Ϊ�����Ե��о����⡣�ҹ��о���Ա���Ƶ����õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)���շ������±��ղ�����SO2���Ʊ������̵������������£�

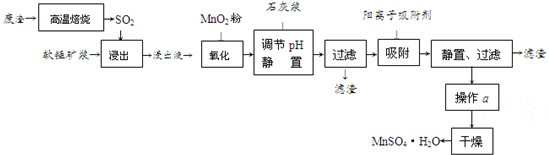
����Һ��pH��2�����еĽ���������Ҫ��Mn2����������������Fe2����Al3����Ca2����Pb2���������������ӡ�
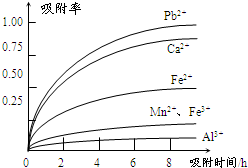
�йؽ������ӵİ뾶�Լ��γ������������ʱ��pH���±��������������������������ӵ�Ч������ͼ��

| ���� | ���Ӱ뾶(pm) | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2�� | 74 | 7.6 | 9.7 |
| Fe3�� | 64 | 2.7 | 3.7 |
| Al3�� | 50 | 3.8 | 4.7 |
| Mn2�� | 80 | 8.3 | 9.8 |
| Pb2�� | 121 | 8.0 | 8.8 |
| Ca2�� | 99 | �� | �� |
��֪PbO2�������Դ���MnO2����ش��������⣺
�� д��������������Ҫ��Ӧ�Ļ�ѧ����ʽ ��������������Ҫ��Ӧ�����ӷ���ʽ ��
�� ���������Һ���м���ʯ�ҽ������ڵ���pH��pHӦ������ ��
�� ���������������ڳ�ȥ���ʽ������ӡ���������������������Ч���������� ����д��ţ���
a����Һ��pH b���������ӵĵ�� c���������ӵİ뾶 d������ʱ��
�� ����a���� �ȹ��̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����SO2���ŷš���������SO2�ǻ�������Ҫ���⡣�ҹ��о���Ա���Ƶ����õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)���շ������±��ղ�����SO2���Ʊ������̵������������£�

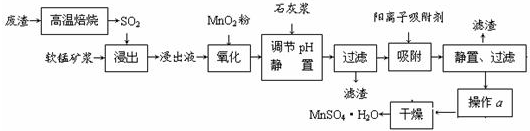
����Һ��pH��2�����еĽ���������Ҫ��Mn2����������������Fe2����Al3����Ca2����Pb2���������������ӡ�
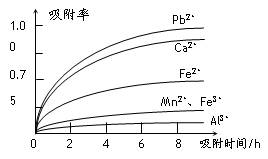
�йؽ������ӵİ뾶�Լ��γ������������ʱ��pH���±��������������������������ӵ�Ч������ͼ��
| ���� | ���Ӱ뾶(pm) | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2�� | 74 | 7.6 | 9.7 |
| Fe3�� | 64 | 2.7 | 3.7 |
| Al3�� | 50 | 3.8 | 4.7 |
| Mn2�� | 80 | 8.3 | 9.8 |
| Pb2�� | 121 | 8.0 | 8.8 |
| Ca2�� | 99 | �� | �� |
��֪PbO2��������ǿ��MnO2����ش��������⣺
�� д��������������Ҫ��Ӧ�Ļ�ѧ����ʽ ��
������������Ҫ��Ӧ�����ӷ���ʽ ��
�� ���������Һ���м���ʯ�ҽ������ڵ���pH��pHӦ��������Χ ��
��������Ҫ�ɷ��� ��
�� ���������������ڳ�ȥ���ʽ������ӡ���������������������Ч����������
����д��ţ���
a����Һ��pH b���������ӵ������� c���������ӵİ뾶 d������ʱ��
�� ����a���� ���̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��ɽ��ʡ�ij�������һ�и߿���ѧģ���Ծ����ţ��������棩 ���ͣ������

| ���� | ���Ӱ뾶��pm�� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 74 | 7.6 | 9.7 |
| Fe3+ | 64 | 2.7 | 3.7 |
| Al3+ | 50 | 3.8 | 4.7 |
| Mn2+ | 80 | 8.3 | 9.8 |
| Pb2+ | 121 | 8.0 | 8.8 |
| Ca2+ | 99 | - | - |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com