
��12�֣���Ȳ����ϩ���ƣ��ڴ�����10%�����5%���ṯ��ˮ��Һ���������£�Ҳ����������ʵ�����ˮ�����ӳɷ�Ӧ��
��1����֪���ǻ���˫��̼ԭ��ֱ����������ϩ���ṹ���ȶ�����ת��Ϊ�ʻ������
����Ȳ��ˮ�ӳɵĻ�ѧ����ʽ�� ��
������װ�������Ȳ��ˮ�ļӳɷ�Ӧ���ӳɲ����ռ���Dװ���С���ʡ�Բ��ּ��Ⱥͼг�������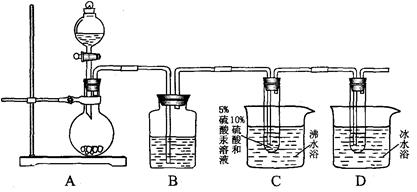
��ʵ�����Ʊ���Ȳ�Ļ�ѧ����ʽ�� ��
��Aװ���Ƴ�����Ȳ�����ŵ���ζ��Ϊ��ȥ��Щ���ʣ�װ��B��ʢ�ŵ��Լ��� ��
��Cװ�ò��÷�ˮԡ�������� ������ĸ����
a�����ڿ��£�ʹ���Ⱦ��� b�����ȷ�Ӧ����ѧ��Ӧ���� c������������
��3��Ϊ����ӳɲ����еĹ����ţ�ѡ���ҩƷ�� ������ĸ����
a�������� b��10%����������Һ c��2%����ͭ��Һ
d��2%��������Һ e������̼������Һ
��������ŵ�ʵ���У�������Ӧ�Ļ�ѧ����ʽ�� ��
(1)CH��CH+H2O
 ��
��
(2)��CaC2+2H2O CH��CH+Ca(OH)2����CuSO4��Һ����a��b��c��
CH��CH+Ca(OH)2����CuSO4��Һ����a��b��c��
(3)b��c����2ml10������������Һ�е���2������ͭ��Һ4~6�Σ���
CH3CHO+2Cu(OH)2+NaOH  CH3COONa+Cu2O��+3H2O��
CH3COONa+Cu2O��+3H2O��
���������������1����Ȳ��ˮ�ӳɵĻ�ѧ����ʽ��CH��CH+H2O
 ����2������ʵ�������õ�ʯ��ˮ��Ӧ���Ʊ���Ȳ�ģ���ѧ����ʽ��CaC2+2H2O
����2������ʵ�������õ�ʯ��ˮ��Ӧ���Ʊ���Ȳ�ģ���ѧ����ʽ��CaC2+2H2O CH��CH+Ca(OH)2����Aװ���Ƴ�����Ȳ��Ϊ����H2S�������ŵ���ζ��Ϊ��ȥ��Щ���ʣ�װ��B��ʢ�ŵ��Լ���CuSO4��Һ����Cװ�ò��÷�ˮԡ��������a�����ڿ��£�ʹ���Ⱦ��ȣ�b�����ȷ�Ӧ����ѧ��Ӧ���ʣ�c�������������3�����ӳɷ�Ӧ�����к���ȩ������Ӧ�������Ƶ�������ͭ����Һ�����飬���ѡ����Լ���b��c����������ŵ�ʵ���У�������Ӧ�Ļ�ѧ����ʽ��CH3CHO+2Cu(OH)2+NaOH
CH��CH+Ca(OH)2����Aװ���Ƴ�����Ȳ��Ϊ����H2S�������ŵ���ζ��Ϊ��ȥ��Щ���ʣ�װ��B��ʢ�ŵ��Լ���CuSO4��Һ����Cװ�ò��÷�ˮԡ��������a�����ڿ��£�ʹ���Ⱦ��ȣ�b�����ȷ�Ӧ����ѧ��Ӧ���ʣ�c�������������3�����ӳɷ�Ӧ�����к���ȩ������Ӧ�������Ƶ�������ͭ����Һ�����飬���ѡ����Լ���b��c����������ŵ�ʵ���У�������Ӧ�Ļ�ѧ����ʽ��CH3CHO+2Cu(OH)2+NaOH  CH3COONa +Cu2O��+3H2O��
CH3COONa +Cu2O��+3H2O��
���㣺������Ȳ��ʵ�����Ʒ������ʼ�����ļ����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������У��ܷ���ȡ����Ӧ����ͬ���칹�����
| A������ | B���Ҵ� | C���������� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ʲ���������ϩ�ӳɲ������
| A��CH3CH3 | B��CH3CHCl2 | C��CH3CH2OH | D��CH3CH2Br |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����˵����ȷ����
| A��ú��������Һ���������������̣��ɱ�Ϊ�����Դ |
| B��ʯ�ͷ���ɻ�����ᡢ������������ |
| C��ͨ��ʯ�͵��ѻ����Եõ���ϩ����ϩ����Ҫ��������ԭ�� |
| D��ú�����ת��Ϊ��¯����ú���͡���̿�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�ʯ�����ִ���ҵ��ѪҺ����ϩ����������Ժ���һ������ʯ�ͻ�����չˮƽ����ش��������⡣
��1�����������У�������ͨ����ϩ�����ӳɷ�Ӧ�õ����� (�����)��
| A��CH3CH3 | B��CH3CHCl2 | C��CH3CH2OH | D��CH3CH2Br |
 2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
2CH3COOH��������ϩΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·������ͼ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�����и����е������л����������ͬ�����ʡ�ͬϵ���ͬ���칹��ȣ����ж�����֮��Ĺ�ϵ
��2��������Ͷ��� ��
��1����ϩ�ͻ����� ��
��2��֧��ֻ��һ���һ���ʽ����С�������Ľṹ��ʽ ��
��3��д����ȩ��Һ��������������Һ���ȵĻ�ѧ����ʽ�� ��
��4��д��1,3-����ϩ���嵥�ʷ���1,4-�ӳɵķ�Ӧ����ʽ
��5��д�����Ҵ�һ����������Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�������һ�����ױ��ж������ױ����������ױ����֣��������ںϳɸ���Ⱦ�ϡ�ij̽��С���������з�Ӧ��װ���Ʊ�һ�����ױ���
ʵ���п����õ������ݣ�
ʵ�鲽�裺�ٰ������1��3����Ũ������Ũ��������40 mL��
��������ƿ�м���15 mL(13g)�ױ�����ͼ��ʾװ��ҩƷ������������
��������ƿ�м�����ᣬ�����Ͻ��裨��������������ȥ����
�ܿ����¶�ԼΪ50�棬��Ӧ��Լ10 min������ƿ���д�������ɫ��״Һ����֣�
�ݷ����һ�����ױ���������13.60 g��
��ش��������⣺
��1�����ƻ���ķ�����________����Ӧ���費�Ͻ��裬Ŀ����____________________
_____________________________��
��2������A��������________ ��ʹ�ø�����ǰ������еIJ�����_________��
��3����ʵ���������ƿ���ռ����IJ�����٣����ܵ�ԭ����_________��
��4�����뷴Ӧ�����ķ������£�
���У�����1������Ϊ________������2����Ҫʹ�����������е�________������ţ���
| A�������� | B���ƾ��� | C���¶ȼ� | D����Һ©�� e�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�����A~F�Ǽ��ֳ������ķ������ģ�͡�
��������ģ�ͣ��ش��������⣺
��1�������º�̼����ߵ�Һ̬���� ��
��2���ܹ������ӳɷ�Ӧ����̬���� ��
��3��һ�ȴ����ͬ���칹�������� ��
��4������ԭ�Ӿ���ͬһƽ���ϵ��� ��
��5����ʹ���Ը��������Һ��ɫ���� �����Ӧ��ĸ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������е��˶�Ա������˻�Ť��ʱ����ҽ�漴���˶�Ա�����˲�λ����ҩ�����������飨�е�Ϊ12.27�棩�����оֲ��䶳����Ӧ��������
��1����ȡ�����飨CH3CH2Cl������õķ�����__________��
| A����������������ȡ����Ӧ |
| B����ϩ�����������ӳɷ�Ӧ |
| C���������Ȼ��ⷴӦ |
| D����ϩ���Ȼ��ⷢ���ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com