
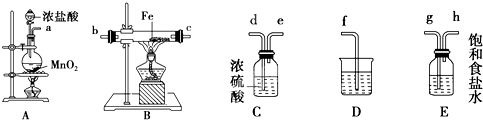
��15�֣�ij����С�齫��ͼ��ʾװ�ð�һ��˳�����ӣ���ʵ��������ȡһ������FeCl3����ͨ�����������ַ�Ӧ����
��ش��������⣺
��1�� A�з�����Ӧ�Ļ�ѧ����ʽΪ______ _________________________________��
_________________________________��
��2����װ�õ���ȷ����˳��Ϊ����дװ�ô��ţ�A��______��______��______��
______D��
��3��װ��C��������________________________________________________��
д��װ��D�з�Ӧ�����ӷ���ʽ___________ _________________________��
_________________________��
��4����Ӧ��ʼ��B��Ӳ�ʲ������ڵ�����Ϊ______________________________ ��
��
���Լ����������к���Fe3+���Լ���____________����д�Լ����ƣ���
(5) ��С��������ͼ��ʾװ���ռ�β������������������������
������ ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ�
ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ�
��Ϊ��߲�����ȷ�ԣ���ͼװ���е�Һ�����________��
�ռ����������ǰӦ���еIJ�����____________��
�������ʼ����ʱ������ȷ��������ʱ�����ұߵζ���Һ�棬 �ᵼ��������������__________���ƫ����ƫС������Ӱ�족����
�ᵼ��������������__________���ƫ����ƫС������Ӱ�족����
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣�ij����С�齫��ͼ��ʾװ�ð�һ��˳�����ӣ���ʵ��������ȡһ������FeCl3����ͨ�����������ַ�Ӧ����
��ش��������⣺
��1�� A�з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
��2�� ��װ�õ���ȷ����˳��Ϊ����дװ�ô��ţ�A��______��______��______��
______D��
��3�� װ��C��������________________________________________________��
д��װ��D�з�Ӧ�����ӷ���ʽ____________________________________��
��4�� ��Ӧ��ʼ��B��Ӳ�ʲ������ڵ�����Ϊ______________________________��
���Լ����������к���Fe3+���Լ���____________����д�Լ����ƣ���
(5) ��С��������ͼ��ʾװ���ռ�β������������������������
�� ����ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ�
�� Ϊ��߲�����ȷ�ԣ���ͼװ���е�Һ�����________��
�ռ����������ǰӦ���еIJ�����____________��
�� �����ʼ����ʱ������ȷ��������ʱ�����ұߵζ���Һ�棬�ᵼ��������������__________���ƫ����ƫС������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡ�����и�����ѧ�������У�һģ����ѧ�Ծ� ���ͣ�ʵ����
��15�֣�ij����С�齫��ͼ��ʾװ�ð�һ��˳�����ӣ���ʵ��������ȡһ������FeCl3����ͨ�����������ַ�Ӧ����

��ش��������⣺
��1�� A�з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
��2�� ��װ�õ���ȷ����˳��Ϊ����дװ�ô��ţ�A��______��______��______��
______D��
��3�� װ��C��������________________________________________________��
д��װ��D�з�Ӧ�����ӷ���ʽ____________________________________��
��4�� ��Ӧ��ʼ��B��Ӳ�ʲ������ڵ�����Ϊ______________________________��
���Լ����������к���Fe3+���Լ���____________����д�Լ����ƣ���
(5) ��С��������ͼ��ʾװ���ռ�β������������������������

�� ����ͼ��ʾ����װ���ɸ���ܡ��齺�ܺ�50 mL�ζ��ܸ������װ���ɣ��˴����õζ�����________�����ʽ����ʽ�����ζ��ܡ�
�� Ϊ��߲�����ȷ�ԣ���ͼװ���е�Һ�����________��
�ռ����������ǰӦ���еIJ�����____________��
�� �����ʼ����ʱ������ȷ��������ʱ�����ұߵζ���Һ�棬�ᵼ��������������__________���ƫ����ƫС������Ӱ�족����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com