
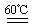 K2CO3+CO2��+2ClO2��+H2O��3�֣�
K2CO3+CO2��+2ClO2��+H2O��3�֣�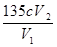 ��3�֣���ƫ�� ƫ�ͣ���1�֣�
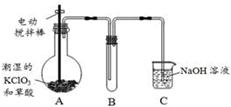
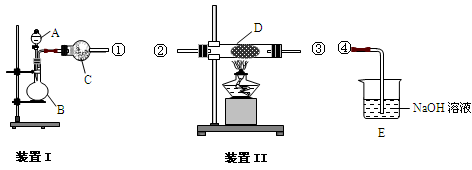
��3�֣���ƫ�� ƫ�ͣ���1�֣� K2CO3+CO2��+2ClO2��+H2O����2��A���������¶ȿ���װ�ã�Ҫ�����¶ȱ���ʹ���¶ȼƲ����¶ȣ���Ӧ��60��ʱ���У�Ӧ��ˮԡ���ȣ����ƾ����⣬����Ҫ�IJ����������ձ����¶ȼƣ�Bװ��Ϊ�������ȵ��ռ�װ�ã��������ȣ�ClO2���ķе�Ϊ11��0�棬������ڱ�ˮԡ�У���ԭ����ʹClO2������������ٻӷ�����3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����NaClO2��Һ���Ƶ�NaClO2����IJ������裺�������ᾧ���ڳ��ȹ��ˣ���ϴ�ӣ��ܸ����4��������100 mL c mol/LNa2S2O3����Һʱ���õ��IJ����������ձ�����Ͳ����������У�100 mL����ƿ����ͷ�ιܣ��ڵζ��������������������ƽ�вⶨ��ԭ���Ǽ������۲���2�з�����ӦΪ����������⻯�������������·�Ӧ���ɵ��ʵ⡢�Ȼ��غ�ˮ�����ӷ���ʽΪ2ClO2 + 10I- + 8H+ = 2Cl- + 5I2 + 4H2O���ܸ��������Ӧ�ù�ϵʽ��ClO2����5Na2S2O3������������ݼ����ClO2��Һ��Ũ��Ϊ
K2CO3+CO2��+2ClO2��+H2O����2��A���������¶ȿ���װ�ã�Ҫ�����¶ȱ���ʹ���¶ȼƲ����¶ȣ���Ӧ��60��ʱ���У�Ӧ��ˮԡ���ȣ����ƾ����⣬����Ҫ�IJ����������ձ����¶ȼƣ�Bװ��Ϊ�������ȵ��ռ�װ�ã��������ȣ�ClO2���ķе�Ϊ11��0�棬������ڱ�ˮԡ�У���ԭ����ʹClO2������������ٻӷ�����3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����NaClO2��Һ���Ƶ�NaClO2����IJ������裺�������ᾧ���ڳ��ȹ��ˣ���ϴ�ӣ��ܸ����4��������100 mL c mol/LNa2S2O3����Һʱ���õ��IJ����������ձ�����Ͳ����������У�100 mL����ƿ����ͷ�ιܣ��ڵζ��������������������ƽ�вⶨ��ԭ���Ǽ������۲���2�з�����ӦΪ����������⻯�������������·�Ӧ���ɵ��ʵ⡢�Ȼ��غ�ˮ�����ӷ���ʽΪ2ClO2 + 10I- + 8H+ = 2Cl- + 5I2 + 4H2O���ܸ��������Ӧ�ù�ϵʽ��ClO2����5Na2S2O3������������ݼ����ClO2��Һ��Ũ��Ϊ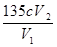 g / L�������ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ���������ı�Һ���ƫ����ⶨ���ƫ�ߣ����ζ���ʼ���Ӷ������ζ��յ�ʱ��ȷ�������������ı�Һ���ƫС����ⶨ���ƫ�͡�
g / L�������ζ�ǰ�ζ��ܼ����������ݣ��ζ���������ʧ���������ı�Һ���ƫ����ⶨ���ƫ�ߣ����ζ���ʼ���Ӷ������ζ��յ�ʱ��ȷ�������������ı�Һ���ƫС����ⶨ���ƫ�͡�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
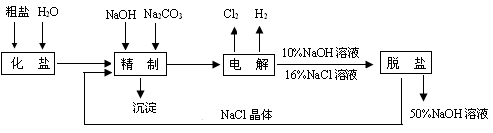
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
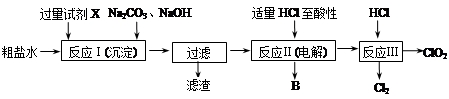
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| | X | Y | Z |
| A | NO2 | SO2 | BaCl2 |
| B | NH3 | CO2 | Al2(SO4)3 |
| C | NH3 | CO2 | CaCl2 |
| D | CO2 | SO2 | CaCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cl2�л�������HCl��ͨ��ʢ����������Һ��ϴ��ƿ |
| B��Cl2�л�������ˮ������ͨ������Ũ���ᣬ�ռ����� |
| C��Cl2�л�������HCl��ͨ����ʯ�Һ��ռ����� |
| D��HCl�л�������Cl2��ͨ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com